Skeletal Muscle Fiber Size and Gene Expression in the Oldest-Old With Differing Degrees of Mobility
- PMID: 30971947
- PMCID: PMC6443969
- DOI: 10.3389/fphys.2019.00313
Skeletal Muscle Fiber Size and Gene Expression in the Oldest-Old With Differing Degrees of Mobility
Erratum in
-
Corrigendum: Skeletal Muscle Fiber Size and Gene Expression in the Oldest-Old With Differing Degrees of Mobility.Front Physiol. 2020 Feb 25;11:127. doi: 10.3389/fphys.2020.00127. eCollection 2020. Front Physiol. 2020. PMID: 32161550 Free PMC article.
Abstract
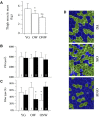
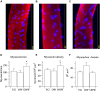
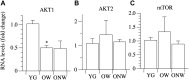
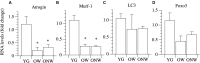
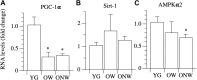
The oldest-old, in the ninth and tenth decades of their life, represent a population characterized by neuromuscular impairment, which often implies a loss of mobility and independence. As recently documented by us and others, muscle atrophy and weakness are accompanied by an unexpected preservation of the size and contractile function of skeletal muscle fibers. This suggests that, while most fibers are likely lost with their respective motoneurons, the surviving fibers are well preserved. Here, we investigated the mechanisms behind this fiber preservation and the relevance of physical activity, by comparing a group of 6 young healthy controls (YG: 22-28 years) with two groups of oldest-old (81-96 years), one able to walk (OW: n = 6, average 86 years) and one confined to a wheelchair (ONW n = 9, average 88 years). We confirmed previous results of fiber preservation and, additionally, observed a shift in fiber type, toward slow predominance in OW and fast predominance in ONW. Myonuclear density was increased in muscles of ONW, compared to YG and OW, potentially indicative of an ongoing atrophy process. We analyzed, by RT-qPCR, the expression of genes relevant for fiber size and type regulation in a biopsy sample from the vastus lateralis. In all oldest-old both myostatin and IGF-1 expression were attenuated compared to YG, however, in ONW two specific IGF-1 isoforms, IGF-1EA and MGF, demonstrated a further significant decrease compared to OW. Surprisingly, atrogenes (MURF1 and atrogin) expression was also significantly reduced compared to YG and this was accompanied by a close to statistically significantly attenuated marker of autophagy, LC3. Among the determinants of the metabolic fiber type, PGC1α was significantly reduced in both OW and ONW compared to YG, while AMPK was down-regulated only in ONW. We conclude that, in contrast to the shift of the balance in favor of pro-atrophy factors found by other studies in older adults (decreased IGF-1, increase of myostatin, increase of atrogenes), in the oldest-old the pro-atrophy factors also appear to be down-regulated, allowing a partial recovery of the proteostasis balance. Furthermore, the impact of muscle activity, as a consequence of lost or preserved walking ability, is limited.
Keywords: aging; gene expression; muscle atrophy; myonuclei; oldest-old; physical activity; single muscle fibers.
Figures

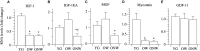
References
-
- Bergen H. R., III, Farr J. N., Vanderboom P. M., Atkinson E. J., White T. A., Singh R. J., et al. (2015). Myostatin as a mediator of sarcopenia versus homeostatic regulator of muscle mass: insights using a new mass spectrometry-based assay. Skelet. Muscle 5:21. 10.1186/s13395-015-0047-45 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

