Cutting-Edge Search for Safer Opioid Pain Relief: Retrospective Review of Salvinorin A and Its Analogs
- PMID: 30971961
- PMCID: PMC6445891
- DOI: 10.3389/fpsyt.2019.00157
Cutting-Edge Search for Safer Opioid Pain Relief: Retrospective Review of Salvinorin A and Its Analogs
Abstract
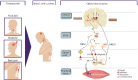
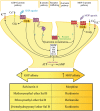
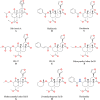
Over the years, pain has contributed to low life quality, poor health, and economic loss. Opioids are very effective analgesic drugs for treating mild, moderate, or severe pain. Therapeutic application of opioids has been limited by short and long-term side effects. These side effects and opioid-overuse crisis has intensified interest in the search for new molecular targets and drugs. The present review focuses on salvinorin A and its analogs with the aim of exploring their structural and pharmacological profiles as clues for the development of safer analgesics. Ethnopharmacological reports and growing preclinical data have demonstrated the antinociceptive effect of salvinorin A and some of its analogs. The pharmacology of analogs modified at C-2 dominates the literature when compared to the ones from other positions. The distinctive binding affinity of these analogs seems to correlate with their chemical structure and in vivo antinociceptive effects. The high susceptibility of salvinorin A to chemical modification makes it an important pharmacological tool for cellular probing and developing analogs with promising analgesic effects. Additional research is still needed to draw reliable conclusions on the therapeutic potential of salvinorin A and its analogs.
Keywords: analgesic; analogs; opioid receptors; salvinorin A; side effects.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

