Clinical Utilization of the FilmArray Meningitis/Encephalitis (ME) Multiplex Polymerase Chain Reaction (PCR) Assay
- PMID: 30972012
- PMCID: PMC6443843
- DOI: 10.3389/fneur.2019.00281
Clinical Utilization of the FilmArray Meningitis/Encephalitis (ME) Multiplex Polymerase Chain Reaction (PCR) Assay
Abstract
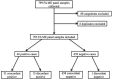
Objective: To assess the clinical utilization and performance of the FilmArray® Meningitis/Encephalitis (ME) multiplex polymerase chain reaction (PCR) panel in a hospital setting. Background: Rapid diagnosis and treatment of central nervous system (CNS) infections are critical to reduce morbidity and mortality. The ME panel is a Food and Drug Administration (FDA) approved rapid multiplex PCR assay that targets 14 bacteria, viruses, and fungi. Previous studies show an overall agreement of 93-99% between the ME panel and conventional diagnostic testing. However, few studies have evaluated the clinical implementation of the ME assay, which is available for routine use at our institution. Methods: We performed a single center retrospective chart review of inpatients who underwent ME panel testing from August 2016 to May 2017. Clinical, radiologic, and laboratory data were reviewed to determine the clinical significance of results. Indication for lumbar puncture (LP), time to results of the ME panel, and duration of antimicrobial therapy were evaluated. Results: Seven hundred and five inpatients underwent ME testing, of whom 480 (68.1%) had clinical suspicion for CNS infection with 416 (59.0%) receiving empiric antimicrobial treatment for CNS infection. The median time-to-result of the ME panel was 1.5 h (IQR, 1.4-1.7). Overall agreement between the ME panel results and clinico-laboratory assessment was 98.2%. Forty-five patients tested positive by ME, of which 12 (26.6%) were determined likely to be clinically insignificant. Conclusions: Routine availability of the ME panel led to overutilization of diagnostic test ordering, as demonstrated by the fact that over one-third of ME panel tests performed were ordered for patients with little or no suspicion for CNS infection. The median time from LP to ME panel result was 1.5 h (IQR, 1.4-1.7). The ME panel's rapid turn-around time contributed to the overuse of the test. Approximately one-quarter of positive ME results were deemed clinically insignificant, though the impact of these positive results requires additional evaluation. Twenty-four and forty-eight hours after the ME panel resulted, 68 and 25% of patients started on empiric therapy remained on antibiotics, respectively. The median time from diagnosis to discontinuation and/or narrowing of antibiotic coverage was 25.6 h (IQR, 3.6-42.5). Further consideration of the appropriate indications for use of the ME panel in clinical settings is required.
Keywords: FilmArray; antibiotic stewardship; encephalitis; meningitis; multiplex PCR.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources