Small-Molecule Immunosuppressive Drugs and Therapeutic Immunoglobulins Differentially Inhibit NK Cell Effector Functions in vitro
- PMID: 30972058
- PMCID: PMC6445861
- DOI: 10.3389/fimmu.2019.00556
Small-Molecule Immunosuppressive Drugs and Therapeutic Immunoglobulins Differentially Inhibit NK Cell Effector Functions in vitro
Abstract
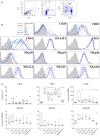
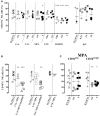
Small-molecule immunosuppressive drugs (ISD) prevent graft rejection mainly by inhibiting T lymphocytes. Therapeutic immunoglobulins (IVIg) are used for substitution, antibody-mediated rejection (AbMR) and HLA-sensitized recipients by targeting distinct cell types. Since the effect of ISD and IVIg on natural killer (NK) cells remains somewhat controversial in the current literature, the aim of this comparative study was to investigate healthy donor's human NK cell functions after exposure to ISD and IVIg, and to comprehensively review the current literature. NK cells were incubated overnight with IL2/IL12 and different doses and combinations of ISD and IVIg. Proliferation was evaluated by 3[H]-thymidine incorporation; phenotype, degranulation and interferon gamma (IFNγ) production by flow cytometry and ELISA; direct NK cytotoxicity by standard 51[Cr]-release and non-radioactive DELFIA assays using K562 as stimulator and target cells; porcine endothelial cells coated with human anti-pig antibodies were used as targets in antibody-dependent cellular cytotoxicity (ADCC) assays. We found that CD69, CD25, CD54, and NKG2D were downregulated by ISD. Proliferation was inhibited by methylprednisolone (MePRD), mycophenolic acid (MPA), and everolimus (EVE). MePRD and MPA reduced degranulation, MPA only of CD56bright NK cells. MePRD and IVIg inhibited direct cytotoxicity and ADCC. Combinations of ISD demonstrated cumulative inhibitory effects. IFNγ production was inhibited by MePRD and ISD combinations, but not by IVIg. In conclusion, IVIg, ISD and combinations thereof differentially inhibit NK cell functions. The most potent drug with an effect on all NK functions was MePRD. The fact that MePRD and IVIg significantly block NK cytotoxicity, especially ADCC, has major implications for AbMR as well as therapeutic strategies targeting cancer and immune cells with monoclonal antibodies.
Keywords: IVIg; cyclosporine A and tacrolimus; cytotoxicity and ADCC; everolimus; human NK cells; immunosuppression; methylprednisolone; mycophenolic acid.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

