Marine sponges of the genus Stelletta as promising drug sources: chemical and biological aspects
- PMID: 30972275
- PMCID: PMC6437601
- DOI: 10.1016/j.apsb.2018.10.003
Marine sponges of the genus Stelletta as promising drug sources: chemical and biological aspects
Abstract
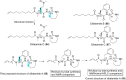
Marine sponges of the genus Stelletta are well known as rich sources of diverse and complex biologically relevant natural products, including alkaloids, terpenoids, peptides, lipids, and steroids. Some of these metabolites, with novel structures and promising biological activities, have attracted a lot of attention from chemists seeking to perform their total synthesis in parallel to intensive biological studies towards new drug leads. In this review, we summarized the distribution of the chemically investigated Stelletta sponges, the isolation, synthesis and biological activities of their secondary metabolites, covering the literature from 1982 to early 2018.
Keywords: Biological activity; Isolation; Marine drug leads; Marine natural products; Stelletta sponge; Total synthesis.
Figures




















References
Publication types
LinkOut - more resources
Full Text Sources

