Prophylactic treatment of hyperbaric oxygen treatment mitigates inflammatory response via mitochondria transfer
- PMID: 30972972
- PMCID: PMC6630002
- DOI: 10.1111/cns.13124
Prophylactic treatment of hyperbaric oxygen treatment mitigates inflammatory response via mitochondria transfer
Abstract
Aims: Hyperbaric oxygen therapy (HBOT) has been widely used as postinjury treatment; however, we investigate its ability to mitigate potential damage as a preconditioning option. Here, we tested the hypothesis that HBOT preconditioning mitigates cell death in primary rat neuronal cells (PRNCs) through the transfer of mitochondria from astrocytes.
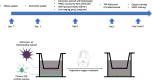
Methods: Primary rat neuronal cells were subjected to a 90-minute HBOT treatment at 2.5 absolute atmospheres prior to either tumor necrosis factor-alpha (TNF-alpha) or lipopolysaccharide (LPS) injury to simulate the inflammation-plagued secondary cell death associated with stroke and traumatic brain injury (TBI). After incubation with TNF-alpha or LPS, the cell viability of each group was examined.
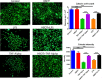
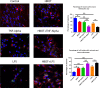
Results: There was a significant increase of cell viability accompanied by mitochondrial transfer in the injury groups that received HBOT preconditioning compared to the injury alone groups (44 ± 5.2 vs 68 ± 4.48, n = 20, P < 0.05). The transfer of mitochondria directly after HBOT treatment was visualized by capturing images in 5-minute intervals, which revealed that the robust transfer of mitochondria begins soon after HBOT and persisted throughout the treatment.
Conclusion: This study shows that HBOT preconditioning stands as a robust prophylactic treatment for sequestration of inflammation inherent in stroke and TBI, possibly facilitating the transfer of resilient mitochondria from astrocytes to inflammation-susceptible neuronal cells in mitigating cell death.
Keywords: hyperbaric; mitochondria transfer; preconditioning; stroke; traumatic brain injury.
© 2019 The Authors. CNS Neuroscience & Therapeutics Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Ma VY, Chan L, Carruthers KJ. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch Phys Med Rehabil. 2014;95(5):986‐995.e1. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

