Applications of molecular engineering in T-cell-based immunotherapies
- PMID: 30972976
- PMCID: PMC7869905
- DOI: 10.1002/wnan.1557
Applications of molecular engineering in T-cell-based immunotherapies
Abstract
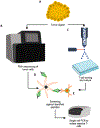
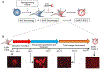
Harnessing an individual's immune cells to mediate antitumor and antiviral responses is a life-saving option for some patients with otherwise intractable forms of cancer and infectious disease. In particular, T-cell-based engineered immune cells are a powerful new class of therapeutics with remarkable efficacy. Clinical experience has helped to define some of the major challenges for reliable, safe, and effective deployment of T-cells against a broad range of diseases. While poised to revolutionize immunotherapy, scalable manufacturing, safety, specificity, and the development of resistance are potential roadblocks in their widespread usage. The development of molecular engineering tools to allow for the direct or indirect engineering of T-cells to enable one to troubleshoot delivery issues, amplify immunomodulatory effects, integrate the synergistic effects of different molecules, and home to the target cells in vivo. In this review, we will analyze thus-far developed cell- and material-based tools for enhancing T-cell therapies, including methods to improve safety and specificity, enhancing efficacy, and overcoming limitations in scalable manufacturing. We summarize the potential of T-cells as immune modulating therapies and the potential future directions for enabling their adoption for a broad range of diseases. This article is categorized under: Nanotechnology Approaches to Biology > Nanoscale Systems in Biology Therapeutic Approaches and Drug Discovery > Nanomedicine for Oncologic Disease Nanotechnology Approaches to Biology > Cells at the Nanoscale.
Keywords: T-cells; adoptive cell transfer; cell engineering; immunotherapy.
© 2019 Wiley Periodicals, Inc.
Figures

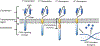

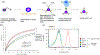


References
-
- Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, … Gabrilovich DI (2000). Clinical significance of defective dendritic cell differentiation in cancer. Clinical Cancer Research, 6(5), 1755–1766. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

