The Retina in Alzheimer's Disease: Histomorphometric Analysis of an Ophthalmologic Biomarker
- PMID: 30973577
- PMCID: PMC6892387
- DOI: 10.1167/iovs.18-25966
The Retina in Alzheimer's Disease: Histomorphometric Analysis of an Ophthalmologic Biomarker
Abstract
Purpose: To provide a histopathologic, morphometric analysis of the retina in Alzheimer's disease (AD).
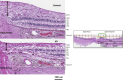
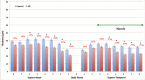
Methods: Human postmortem retinas from eight patients with AD (mean age: 80 ± 12.7 years) and from 11 age-matched controls (mean age: 78 ± 16.57 years) were analyzed. The retinas were sampled from the superior quadrant on both the temporal and nasal sides with respect to the optic nerve. Thickness of the inner and outer layers involving the retinal nerve fiber layer (RNFL), retinal ganglion cell layer (RGCL), inner plexiform layer (IPL), inner nuclear layer (INL), and outer nuclear layer (ONL) were measured and compared between controls and AD. A total of 16 measurements of retinal thickness were acquired for each layer.
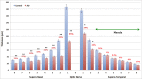
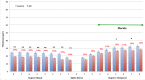
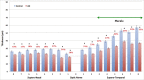
Results: RNFL thinning supero-temporally was significant closest to the optic nerve (∼35% thickness reduction; P < 0.001). Supero-nasally, RNFL was thinner throughout all points (∼40% reduction; P < 0.001). Supero-temporally, RGCL thinning was pronounced toward the macula (∼35% thickness reduction; P < 0.001). Supero-nasally, RGCL showed uniform thinning throughout (∼35% reduction; P < 0.001). IPL thinning supero-temporally was statistically significant in the macula (∼15% reduction; P < 0.01). Supero-nasal IPL featured uniform thinning throughout (∼25% reduction; P < 0.001). Supero-temporally, INL and ONL thinning were pronounced toward the macula (∼25% reduction; P < 0.01). Supero-nasally, INL and ONL were thinner throughout (∼25% reduction; P < 0.01).
Conclusions: Our study revealed marked thinning in both the inner and outer layers of the retina. These quantified histopathologic findings provide a more comprehensive understanding of the retina in AD than previously reported.
Figures





References
-
- Batsch NL, Mittelman MS. World Alzheimer Report 2012: Overcoming the Stigma of Dementia. London: Alzheimer's Disease International; 2012. p. 75.
-
- Uhlmann RF, Larson EB, Koepsell TD, Rees TS, Duckert LG. Visual impairment and cognitive dysfunction in Alzheimer's disease. J Gen Intern Med. 1991;6:126–132. - PubMed

