FOXO3 directly regulates an autophagy network to functionally regulate proteostasis in adult neural stem cells
- PMID: 30973875
- PMCID: PMC6478346
- DOI: 10.1371/journal.pgen.1008097
FOXO3 directly regulates an autophagy network to functionally regulate proteostasis in adult neural stem cells
Abstract
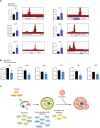
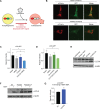
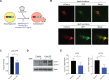
Maintenance of a healthy proteome is essential for cellular homeostasis and loss of proteostasis is associated with tissue dysfunction and neurodegenerative disease. The mechanisms that support proteostasis in healthy cells and how they become defective during aging or in disease states are not fully understood. Here, we investigate the transcriptional programs that are essential for neural stem and progenitor cell (NSPC) function and uncover a program of autophagy genes under the control of the transcription factor FOXO3. Using genomic approaches, we observe that FOXO3 directly binds a network of target genes in adult NSPCs that are involved in autophagy, and find that FOXO3 functionally regulates induction of autophagy in these cells. Interestingly, in the absence of FOXO activity, aggregates accumulate in NSPCs, and this effect is reversed by TOR (target of rapamycin) inhibition. Surprisingly, enhancing FOXO3 causes nucleation of protein aggregates, but does not increase their degradation. The work presented here identifies a genomic network under the direct control of a key transcriptional regulator of aging that is critical for maintaining a healthy mammalian stem cell pool to support lifelong neurogenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

