The highly selective dopamine D3R antagonist, R-VK4-40 attenuates oxycodone reward and augments analgesia in rodents
- PMID: 30974107
- PMCID: PMC6745270
- DOI: 10.1016/j.neuropharm.2019.04.003
The highly selective dopamine D3R antagonist, R-VK4-40 attenuates oxycodone reward and augments analgesia in rodents
Abstract
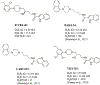
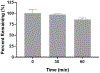
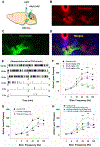
Prescription opioid abuse is a global crisis. New treatment strategies for pain and opioid use disorders are urgently required. We evaluated the effects of R-VK4-40, a highly selective dopamine (DA) D3 receptor (D3R) antagonist, on the rewarding and analgesic effects of oxycodone, the most commonly abused prescription opioid, in rats and mice. Systemic administration of R-VK4-40 dose-dependently inhibited oxycodone self-administration and shifted oxycodone dose-response curves downward in rats. Pretreatment with R-VK4-40 also dose-dependently lowered break-points for oxycodone under a progressive-ratio schedule. To determine whether a DA-dependent mechanism underlies the impact of D3 antagonism in reducing opioid reward, we used optogenetic approaches to examine intracranial self-stimulation (ICSS) maintained by optical activation of ventral tegmental area (VTA) DA neurons in DAT-Cre mice. Photoactivation of VTA DA in non-drug treated mice produced robust ICSS behavior. Lower doses of oxycodone enhanced, while higher doses inhibited, optical ICSS. Pretreatment with R-VK4-40 blocked oxycodone-enhanced brain-stimulation reward. By itself, R-VK4-40 produced a modest dose-dependent reduction in optical ICSS. Pretreatment with R-VK4-40 did not compromise the antinociceptive effects of oxycodone in rats, and R-VK4-40 alone produced mild antinociceptive effects without altering open-field locomotion or rotarod locomotor performance. Together, these findings suggest R-VK4-40 may permit a lower dose of prescription opioids for pain management, potentially mitigating tolerance and dependence, while diminishing reward potency. Hence, development of R-VK4-40 as a therapy for the treatment of opioid use disorders and/or pain is currently underway. This article is part of the Special Issue entitled 'New Vistas in Opioid Pharmacology'.
Keywords: Brain-stimulation reward; D(3) receptor antagonist; Opioid analgesia; Oxycodone; R-VK4-40; Self-administration.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures

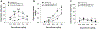
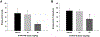

References
-
- Andreoli M, Tessari M, Pilla M, Valerio E, Hagan JJ, Heidbreder CA. 2003. Selective antagonism at dopamine D3 receptors prevents nicotine-triggered relapse to nicotineseeking behavior. Neuropsychopharmacology 28: 1272–80. - PubMed
-
- Ashby CR Jr., Paul M, Gardner EL, Heidbreder CA, Hagan JJ 2003. Acute administration of the selective D3 receptor antagonist SB-277011A blocks the acquisition and expression of the conditioned place preference response to heroin in male rats. Synapse 48: 154–6. - PubMed
-
- Aujla H, Beninger RJ. 2005. The dopamine D(3) receptor-preferring partial agonist BP 897 dose-dependently attenuates the expression of amphetamine-conditioned place preference in rats. Behav Pharmacol 16: 181–6. - PubMed
-
- Bauco P, Wise RA. 1997. Synergistic effects of cocaine with lateral hypothalamic brain stimulation reward: lack of tolerance or sensitization. J Pharmacol Exp Ther 283: 1160–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

