Necrotizing Enterocolitis, Gut Microbiota, and Brain Development: Role of the Brain-Gut Axis
- PMID: 30974443
- PMCID: PMC6604259
- DOI: 10.1159/000497420
Necrotizing Enterocolitis, Gut Microbiota, and Brain Development: Role of the Brain-Gut Axis
Abstract
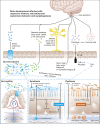
Necrotizing enterocolitis (NEC) is a relatively common disease in very-low-birth-weight infants and is associated with high mortality and morbidity. In survivors, neurodevelopmental impairment is frequently seen. The exact etiology remains largely to be elucidated, but microbiota are considered to play a major role in the development of NEC. Furthermore, emerging evidence exists that the microbiota is also of importance in brain function and development. Therefore, microbiota characterization has not only potential as a diagnostic or even preventive tool to predict NEC, but may also serve as a biomarker to monitor and possibly even as a target to manipulate brain development. Analysis of fecal volatile organic compounds, which shape the volatile metabolome and reflect microbiota function and host interaction, has been shown to be of interest in the diagnosis of NEC and late-onset sepsis. In this review, we discuss evidence of the role of the complex interplay between microbiota, NEC, and brain development, including the brain-gut axis in preterm infants.
Keywords: Brain development; Brain-gut axis; Necrotizing enterocolitis; Preterm infants.
© 2019 The Author(s) Published by S. Karger AG, Basel.
Figures
Similar articles
-
Unraveling the Microbiome of Necrotizing Enterocolitis: Insights in Novel Microbial and Metabolomic Biomarkers.Microbiol Spectr. 2021 Oct 31;9(2):e0117621. doi: 10.1128/Spectrum.01176-21. Epub 2021 Oct 27. Microbiol Spectr. 2021. PMID: 34704805 Free PMC article.
-
The potential of gut microbiota and fecal volatile organic compounds analysis as early diagnostic biomarker for necrotizing enterocolitis and sepsis in preterm infants.Expert Rev Gastroenterol Hepatol. 2018 May;12(5):457-470. doi: 10.1080/17474124.2018.1446826. Epub 2018 Mar 6. Expert Rev Gastroenterol Hepatol. 2018. PMID: 29488419 Review.
-
The Microbiome and Metabolome of Preterm Infant Stool Are Personalized and Not Driven by Health Outcomes, Including Necrotizing Enterocolitis and Late-Onset Sepsis.mSphere. 2018 Jun 6;3(3):e00104-18. doi: 10.1128/mSphere.00104-18. Print 2018 Jun 27. mSphere. 2018. PMID: 29875143 Free PMC article.
-
Longitudinal fecal microbiota and volatile metabolomics preceding necrotizing enterocolitis in preterm infants: a case-control study.Sci Rep. 2025 Mar 26;15(1):10419. doi: 10.1038/s41598-025-94692-w. Sci Rep. 2025. PMID: 40140438 Free PMC article.
-
The role of the preterm intestinal microbiome in sepsis and necrotising enterocolitis.Early Hum Dev. 2019 Nov;138:104854. doi: 10.1016/j.earlhumdev.2019.104854. Epub 2019 Aug 31. Early Hum Dev. 2019. PMID: 31481262 Review.
Cited by
-
Unraveling the Microbiome of Necrotizing Enterocolitis: Insights in Novel Microbial and Metabolomic Biomarkers.Microbiol Spectr. 2021 Oct 31;9(2):e0117621. doi: 10.1128/Spectrum.01176-21. Epub 2021 Oct 27. Microbiol Spectr. 2021. PMID: 34704805 Free PMC article.
-
The Regulation of Host Intestinal Microbiota by Polyphenols in the Development and Prevention of Chronic Kidney Disease.Front Immunol. 2020 Jan 8;10:2981. doi: 10.3389/fimmu.2019.02981. eCollection 2019. Front Immunol. 2020. PMID: 31969882 Free PMC article. Review.
-
The gut-brain ConNECtion: exploring the developmental impact of necrotizing enterocolitis on the neonatal brain.Pediatr Res. 2025 Aug 21. doi: 10.1038/s41390-025-04092-z. Online ahead of print. Pediatr Res. 2025. PMID: 40841674 Review.
-
Gastrointestinal Dysbiosis in Neuro-Critically Ill Patients: A Systematic Review of Case-Control Studies.Cureus. 2023 Dec 21;15(12):e50923. doi: 10.7759/cureus.50923. eCollection 2023 Dec. Cureus. 2023. PMID: 38259358 Free PMC article. Review.
-
Oropharyngeal administration of colostrum targeting gut microbiota and metabolites in very preterm infants: protocol for a multicenter randomized controlled trial.BMC Pediatr. 2023 Oct 16;23(1):508. doi: 10.1186/s12887-023-04346-x. BMC Pediatr. 2023. PMID: 37845612 Free PMC article.
References
-
- Yee WH, Soraisham AS, Shah VS, Aziz K, Yoon W, Lee SK, Canadian Neonatal Network Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics. 2012 Feb;129((2)):e298–304. - PubMed
-
- Hickey M, Georgieff M, Ramel S. Neurodevelopmental outcomes following necrotizing enterocolitis. Semin Fetal Neonatal Med. 2018 Dec;23((6)):426–32. - PubMed
-
- Neu J. Necrotizing enterocolitis: the mystery goes on. Neonatology. 2014;106((4)):289–95. - PubMed
-
- Niemarkt HJ, de Meij TG, van de Velde ME, van der Schee MP, van Goudoever JB, Kramer BW, et al. Necrotizing enterocolitis: a clinical review on diagnostic biomarkers and the role of the intestinal microbiota. Inflamm Bowel Dis. 2015 Feb;21((2)):436–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

