Bio-Based Polymers with Potential for Biodegradability
- PMID: 30974537
- PMCID: PMC6432354
- DOI: 10.3390/polym8070262
Bio-Based Polymers with Potential for Biodegradability
Abstract
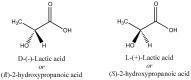
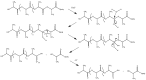
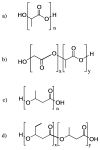
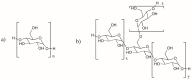
A variety of renewable starting materials, such as sugars and polysaccharides, vegetable oils, lignin, pine resin derivatives, and proteins, have so far been investigated for the preparation of bio-based polymers. Among the various sources of bio-based feedstock, vegetable oils are one of the most widely used starting materials in the polymer industry due to their easy availability, low toxicity, and relative low cost. Another bio-based plastic of great interest is poly(lactic acid) (PLA), widely used in multiple commercial applications nowadays. There is an intrinsic expectation that bio-based polymers are also biodegradable, but in reality there is no guarantee that polymers prepared from biorenewable feedstock exhibit significant or relevant biodegradability. Biodegradability studies are therefore crucial in order to assess the long-term environmental impact of such materials. This review presents a brief overview of the different classes of bio-based polymers, with a strong focus on vegetable oil-derived resins and PLA. An entire section is dedicated to a discussion of the literature addressing the biodegradability of bio-based polymers.
Keywords: PLA; bio-based polymers; biodegradability; vegetable oil-based polymers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Goodman S.H. In: Handbook of Thermoset Plastics. 3rd ed. Dodiuk H., Goodman S.H., editors. William Andrew Publishing; Westwood, MA, USA: 1999. p. 1.
-
- Williams C.K., Hillmyer M.A. Polymers from renewable resources: A perspective for a special issue of polymer reviews. Polym. Rev. 2008;48:1–10. doi: 10.1080/15583720701834133. - DOI
-
- Xia Y., Larock R.C. Vegetable oil-based polymeric materials: Synthesis, properties, and applications. Green Chem. 2010;12:1893–1909. doi: 10.1039/c0gc00264j. - DOI
-
- Gandini A. The irruption of polymers from renewable resources on the scene of macromolecular science and technology. Green Chem. 2011;13:1061–1083. doi: 10.1039/c0gc00789g. - DOI
Publication types
LinkOut - more resources
Full Text Sources

