Sustainable Phenolic Fractions as Basis for Furfuryl Alcohol-Based Co-Polymers and Their Use as Wood Adhesives
- PMID: 30974673
- PMCID: PMC6431995
- DOI: 10.3390/polym8110396
Sustainable Phenolic Fractions as Basis for Furfuryl Alcohol-Based Co-Polymers and Their Use as Wood Adhesives
Abstract
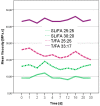
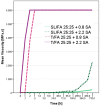
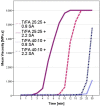
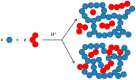
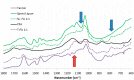
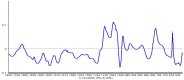
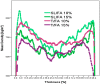
Furfuryl alcohol is a very interesting green molecule used in the production of biopolymers. In the present paper, the copolymerization in acid environment with natural, easily-available, phenolic derivatives is investigated. The processes of polymerization of the furfuryl alcohol with: (i) spent-liquor from the pulping industry and (ii) commercial tannin from acacia mimosa were investigated though viscometry and IR-spectroscopy. The curing kinetics of the formulations highlighted the importance of the amount of furfuryl alcohol and catalyst as well as the effect of temperature for both phenolic-furanic polymers. Evidence of covalent copolymerization has been observed through infrared spectrometry (FT-IR) combined with principal component analysis (PCA) and confirmed with additional solubility tests. These bio-based formulations were applied as adhesives for solid wood and particleboards with interesting results: at 180 °C, the spent-liquor furanic formulations allow wood bonding slightly with lower performance than PVA in dry conditions, while mixed formulations allow the gluing of particleboard with only satisfactory internal bonding tests.
Keywords: adhesive; copolymer; furanic; lignin; multivariate data analysis; panels; poly-furfuryl alcohol; tannin; wood bonding.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Gandini A. Polymers from renewable resources: A challenge for the future of macromolecular materials. Macromolecules. 2008;41:9491–9504. doi: 10.1021/ma801735u. - DOI
-
- Gandini A. Furans as offspring of sugars and polysaccharides and progenitors of a family of remarkable polymers: A review of recent progress. Polym. Chem. 2010;1:245–251. doi: 10.1039/B9PY00233B. - DOI
-
- Deka H., Misra M., Mohanty A. Renewable resource based “all green composites” from kenaf biofiber and poly (furfuryl alcohol) bioresin. Ind. Crop. Prod. 2013;41:94–101. doi: 10.1016/j.indcrop.2012.03.037. - DOI
-
- Gutiérrez T., Buszko M.L., Ingram L.O., Preston J.F. Biotechnology for Fuels and Chemicals. Humana Press; Totowa, NJ, USA: 2002. Reduction of furfural to furfuryl alcohol by ethanologenic strains of bacteria and its effect on ethanol production from xylose. - PubMed
-
- Nagaraja B.M., Kumar V.S., Shasikala V., Padmasri A.H., Sreedhar B., Raju B.D., Rao K.R. A highly efficient Cu/MgO catalyst for vapour phase hydrogenation of furfural to furfuryl alcohol. Catal. Commun. 2003;4:287–293. doi: 10.1016/S1566-7367(03)00060-8. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

