Introduction to the Molecules Special Edition Entitled ' Heparan Sulfate and Heparin: Challenges and Controversies': Some Outstanding Questions in Heparan Sulfate and Heparin Research
- PMID: 30974725
- PMCID: PMC6479682
- DOI: 10.3390/molecules24071399
Introduction to the Molecules Special Edition Entitled ' Heparan Sulfate and Heparin: Challenges and Controversies': Some Outstanding Questions in Heparan Sulfate and Heparin Research
Abstract
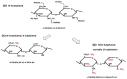
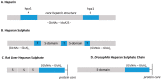
The scope of this article is to provide a brief general introduction to heparan sulfate (HS) and heparin, and attempt to identify some of the central challenges regarding research into the chemistry and biology of glycosaminoglycans (GAGs), some of which are the subject of contributions to the special issue of Molecules (published in volume 23, 2018) entitled 'Heparan Sulfate and Heparin: Challenges and Controversies' [...].
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

