Distinct Difference in Sensitivity of NIR vs. IR Bands of Melamine to Inter-Molecular Interactions with Impact on Analytical Spectroscopy Explained by Anharmonic Quantum Mechanical Study
- PMID: 30974741
- PMCID: PMC6479474
- DOI: 10.3390/molecules24071402
Distinct Difference in Sensitivity of NIR vs. IR Bands of Melamine to Inter-Molecular Interactions with Impact on Analytical Spectroscopy Explained by Anharmonic Quantum Mechanical Study
Abstract
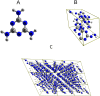
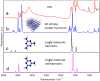
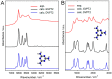
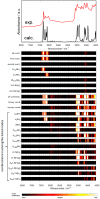
Melamine (IUPAC: 1,3,5-Triazine-2,4,6-triamine) attracts high attention in analytical vibrational spectroscopy due to its misuse as a food adulterant. Vibrational spectroscopy [infrared (IR) and Raman and near-infrared (NIR) spectroscopy] is a major quality control tool in the detection and quantification of melamine content. The physical background for the measured spectra is not interpreted in analytical spectroscopy using chemometrics. In contrast, quantum mechanical calculations are capable of providing deep and independent insights therein. So far, the NIR region of crystalline melamine has not been studied by quantum mechanical calculations, while the investigations of its IR spectra have remained limited. In the present work, we employed fully anharmonic calculation of the NIR spectrum of melamine based on finite models, and also performed IR spectral simulation by using an infinite crystal model-periodic in three dimensions. This yielded detailed and unambiguous NIR band assignments and revised the previously known IR band assignments. We found that the out-of-plane fundamental transitions, which are essential in the IR region, are markedly more sensitive to out-of-plane inter-molecular interactions of melamine than NIR transitions. Proper description of the chemical surrounding of the molecule of melamine is more important than the anharmonicity of its vibrations. In contrast, the NIR bands mostly arise from in-plane vibrations, and remain surprisingly insensitive to the chemical environment. These findings explain previous observations that were reported in IR and NIR analytical studies of melamine.
Keywords: FT-IR; NIR spectroscopy; anharmonic calculation; combination bands; melamine; overtones; quantum chemical calculation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Deim H., Matthias G., Wagner R.A. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH; Weinheim, Germany: 2012. Amino Resins.
-
- Ogawa A. Effect of a melamine resin admixture on the properties of concrete. Concr. J. (Tokyo 1963) 1973;11:12–21. doi: 10.14826/coj1963.11.12_12. - DOI
-
- Ashford R.D., editor. Ashford’s Dictionary of Industrial Chemicals. 3rd ed. Wavelength Publications Ltd.; London, UK: 2011. p. 5713.
-
- Zhong Y., Jaidann M., Zhang Y., Zhang G., Liu H., Ionescu M., Li R., Sun X., Abou-Rachid H., Lussier L.-S. Synthesis of high nitrogen doping of carbon nanotubes and modeling the stabilization of filled DAATO@CNTs (10,10) for nanoenergetic materials. J. Phys. Chem. Solids. 2010;71:134–139. doi: 10.1016/j.jpcs.2009.07.030. - DOI
-
- Hauck R.D., Stephenson H.F. Nitrification of triazine nitrogen. Fertilizer Nitrogen Sources. 1964;12:147–151. doi: 10.1021/jf60132a014. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

