MAPK/ERK Signaling in Regulation of Renal Differentiation
- PMID: 30974877
- PMCID: PMC6479953
- DOI: 10.3390/ijms20071779
MAPK/ERK Signaling in Regulation of Renal Differentiation
Abstract
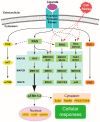
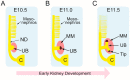
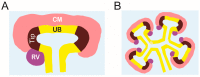
Congenital anomalies of the kidney and urinary tract (CAKUT) are common birth defects derived from abnormalities in renal differentiation during embryogenesis. CAKUT is the major cause of end-stage renal disease and chronic kidney diseases in children, but its genetic causes remain largely unresolved. Here we discuss advances in the understanding of how mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) activity contributes to the regulation of ureteric bud branching morphogenesis, which dictates the final size, shape, and nephron number of the kidney. Recent studies also demonstrate that the MAPK/ERK pathway is directly involved in nephrogenesis, regulating both the maintenance and differentiation of the nephrogenic mesenchyme. Interestingly, aberrant MAPK/ERK signaling is linked to many cancers, and recent studies suggest it also plays a role in the most common pediatric renal cancer, Wilms' tumor.
Keywords: MAPK/ERK signaling; differentiation; extracellular signal-regulated kinase; intracellular signaling; kidney development; nephrogenesis; progenitor cells; self-renewal; ureteric bud branching morphogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Erk MAP kinase regulates branching morphogenesis in the developing mouse kidney.Development. 2001 Nov;128(21):4329-38. doi: 10.1242/dev.128.21.4329. Development. 2001. PMID: 11684667
-
Comparative whole-genome transcriptome analysis in renal cell populations reveals high tissue specificity of MAPK/ERK targets in embryonic kidney.BMC Biol. 2022 May 13;20(1):112. doi: 10.1186/s12915-022-01309-z. BMC Biol. 2022. PMID: 35550069 Free PMC article.
-
Omics profiling identifies the regulatory functions of the MAPK/ERK pathway in nephron progenitor metabolism.Development. 2022 Oct 1;149(19):dev200986. doi: 10.1242/dev.200986. Epub 2022 Oct 3. Development. 2022. PMID: 36189831 Free PMC article.
-
Renal branching morphogenesis: morphogenetic and signaling mechanisms.Semin Cell Dev Biol. 2014 Dec;36:2-12. doi: 10.1016/j.semcdb.2014.07.011. Epub 2014 Jul 28. Semin Cell Dev Biol. 2014. PMID: 25080023 Review.
-
Renin-angiotensin system in ureteric bud branching morphogenesis: implications for kidney disease.Pediatr Nephrol. 2014 Apr;29(4):609-20. doi: 10.1007/s00467-013-2616-3. Epub 2013 Sep 7. Pediatr Nephrol. 2014. PMID: 24061643 Review.
Cited by
-
Profiling of the serum MiRNAome in pediatric egyptian patients with wilms tumor.Front Mol Biosci. 2024 Oct 15;11:1453562. doi: 10.3389/fmolb.2024.1453562. eCollection 2024. Front Mol Biosci. 2024. PMID: 39473825 Free PMC article.
-
Integrating network analysis and experimental validation to reveal the mechanism of si-jun-zi decoction in the treatment of renal fibrosis.Heliyon. 2024 Aug 2;10(16):e35489. doi: 10.1016/j.heliyon.2024.e35489. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39220912 Free PMC article.
-
Oxidative stress in intervertebral disc degeneration: Molecular mechanisms, pathogenesis and treatment.Cell Prolif. 2023 Sep;56(9):e13448. doi: 10.1111/cpr.13448. Epub 2023 Mar 13. Cell Prolif. 2023. PMID: 36915968 Free PMC article. Review.
-
Developmental and tissue-specific roles of mammalian centrosomes.FEBS J. 2025 Feb;292(4):709-726. doi: 10.1111/febs.17212. Epub 2024 Jun 27. FEBS J. 2025. PMID: 38935637 Free PMC article. Review.
-
Anti‑oncogenic and pro‑myogenic action of the MKK6/p38/AKT axis induced by targeting MEK/ERK in embryonal rhabdomyosarcoma.Oncol Rep. 2022 Sep;48(3):151. doi: 10.3892/or.2022.8363. Epub 2022 Jul 8. Oncol Rep. 2022. PMID: 35801577 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

