Heterologous Expression of a Thermostable α-Glucosidase from Geobacillus sp. Strain HTA-462 by Escherichia coli and Its Potential Application for Isomaltose⁻Oligosaccharide Synthesis
- PMID: 30974879
- PMCID: PMC6479687
- DOI: 10.3390/molecules24071413
Heterologous Expression of a Thermostable α-Glucosidase from Geobacillus sp. Strain HTA-462 by Escherichia coli and Its Potential Application for Isomaltose⁻Oligosaccharide Synthesis
Abstract
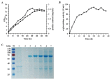
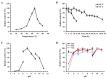
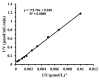
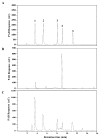
Isomaltose-oligosaccharides (IMOs), as food ingredients with prebiotic functionality, can be prepared via enzymatic synthesis using α-glucosidase. In the present study, the α-glucosidase (GSJ) from Geobacillus sp. strain HTA-462 was cloned and expressed in Escherichia coli BL21 (DE3). Recombinant GSJ was purified and biochemically characterized. The optimum temperature condition of the recombinant enzyme was 65 °C, and the half-life was 84 h at 60 °C, whereas the enzyme was active over the range of pH 6.0-10.0 with maximal activity at pH 7.0. The α-glucosidase activity in shake flasks reached 107.9 U/mL and using 4-Nitrophenyl β-D-glucopyranoside (pNPG) as substrate, the Km and Vmax values were 2.321 mM and 306.3 U/mg, respectively. The divalent ions Mn2+ and Ca2+ could improve GSJ activity by 32.1% and 13.8%. Moreover, the hydrolysis ability of recombinant α-glucosidase was almost the same as that of the commercial α-glucosidase (Bacillus stearothermophilus). In terms of the transglycosylation reaction, with 30% maltose syrup under the condition of 60 °C and pH 7.0, IMOs were synthesized with a conversion rate of 37%. These studies lay the basis for the industrial application of recombinant α-glucosidase.
Keywords: heterologous expression; isomaltose-oligosaccharides; thermostability; transglycosylation; α-glucosidase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Huang Z.H., Li Z.H., Su Y.J., Zhu Y.F., Zeng W., Chen G.G., Liang Z.Q. Continuous production of isomalto-oligosaccharides by thermo-inactivated cells of Aspergillus niger J2 with coarse perlite as an immobilizing material. Appl. Biochem. Biotechnol. 2018;185:1088–1099. doi: 10.1007/s12010-018-2706-6. - DOI - PubMed
-
- Shi Q., Hou Y.X., Juvonen M., Tuomainen P., Kajala I., Shukla S., Goyal A., Maaheimo H., Katina K., Tenkanen M. Optimization of isomaltooligosaccharide size distribution by acceptor reaction of Weissella confusa dextransucrase and characterization of novel α-(1→2)-branched isomaltooligosaccharides. J. Agric. Food Chem. 2016;64:3276–3286. doi: 10.1021/acs.jafc.6b01356. - DOI - PubMed
-
- Ojha S., Mishra S., Chand S. Production of isomalto-oligosaccharides by cell bound α-glucosidase of Microbacterium sp. LWT-Food Sci. Technol. 2015;60:486–494. doi: 10.1016/j.lwt.2014.08.009. - DOI
-
- Soto-Maldonado C., Concha-Olmos J., Cáceres-Escobar G., Meneses-Gómez P. Sensory evaluation and glycaemic index of a food developed with flour from whole (pulp and peel) overripe banana (Musa cavendishii) discards. LWT-Food Sci. Technol. 2018;92:569–575. doi: 10.1016/j.lwt.2018.03.011. - DOI
-
- Meyer T.S.M., Miguel Â.S.M., Fernández D.E.R., Ortiz G.M.D. Food Production and Industry. IntechOpen; Rijeka, Croatia: 2015. Biotechnological production of oligosaccharides-applications in the food industry. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

