Concepts of Catalysis in Site-Selective Protein Modifications
- PMID: 30974939
- PMCID: PMC6535719
- DOI: 10.1021/jacs.8b13187
Concepts of Catalysis in Site-Selective Protein Modifications
Abstract
The manipulation and modulation of biomolecules has the potential to herald new modes of Biology and Medicine through chemical "editing". Key to the success of such processes will be the selectivities, reactivities and efficiencies that may be brought to bear in bond-formation and bond-cleavage in a benign manner. In this Perspective, we use select examples, primarily from our own research, to examine the current opportunities, limitations and the particular potential of metal-mediated processes as exemplars of possible alternative catalytic modes and manifolds to those already found in nature.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
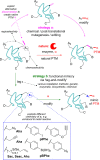
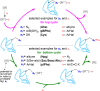
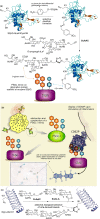
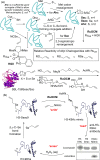

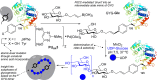
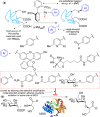
References
-
- Zioudrou C.; Wilchek M.; Patchornik A. Conversion of the L-Serine Residue to an L-Cysteine Residue in Peptides*. Biochemistry 1965, 4, 1811.10.1021/bi00885a018. - DOI
-
- Polgar L.; Bender M. L. A New Enzyme Containing a Synthetically Formed Active Site. Thiol-Subtilisin 1. J. Am. Chem. Soc. 1966, 88, 3153.10.1021/ja00965a060. - DOI
-
- Wu Z. P.; Hilvert D. Conversion of a protease into an acyl transferase: selenolsubtilisin. J. Am. Chem. Soc. 1989, 111, 4513.10.1021/ja00194a064. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

