Laboratory Evaluation of Linearity, Repeatability, and Hematocrit Interference With an Internet-Enabled Blood Glucose Meter
- PMID: 30974988
- PMCID: PMC6501519
- DOI: 10.1177/1932296819841357
Laboratory Evaluation of Linearity, Repeatability, and Hematocrit Interference With an Internet-Enabled Blood Glucose Meter
Abstract
Background: In recent clinical trials, use of the MyGlucoHealth blood glucose meter (BGM) and electronic diary was associated with an unusual reporting pattern of glycemic data and hypoglycemic events. Therefore, the performance of representative BGMs used by the patients was investigated to assess repeatability, linearity, and hematocrit interference in accordance with regulatory guidelines.
Method: Ten devices and 6 strip lots were selected using standard randomization and repeatability procedures. Venous heparinized blood was drawn from healthy subjects, immediately aliquoted and adjusted to 5 target blood glucose (BG) ranges for the repeatability and 11 BG concentrations for the linearity tests. For the hematocrit interference test, each sample within 5 target BG ranges was split into 5 aliquots and adjusted to hematocrit levels across the acceptance range. YSI 2300 STAT Plus was used as the laboratory reference method in all experiments.
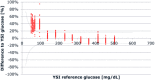
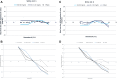
Results: Measurement repeatability or precision was acceptable across the target BG ranges for all devices and strip lots with coefficient of variation (CV) between 3.4-9.7% (mean: 5.7%). Linearity was shown by a correlation coefficient of .991; however, a positive bias was seen for BG <100 mg/dL (86% measurements did not meet ISO15197:2015 acceptance criteria). Significant hematocrit interference (up to 20%) was observed for BG >100 mg/dL (ISO15197:2015 acceptance criteria: ±10%), while the results were acceptable for BG <100 mg/dL.
Conclusions: The BGM met repeatability requirements but demonstrated a significant measurement bias in the low BG range. In addition, it failed the ISO15197:2015 criteria for hematocrit interference.
Keywords: blood glucose meter; hematocrit interference; linearity; repeatability.
Conflict of interest statement
Figures
Comment in
-
Postmarket Surveillance of Blood Glucose Monitor Systems Is Needed for Safety of Subjects and Accurate Determination of Effectiveness in Clinical Trials of Diabetes Drugs and Devices.J Diabetes Sci Technol. 2019 May;13(3):419-423. doi: 10.1177/1932296819843398. Epub 2019 Apr 11. J Diabetes Sci Technol. 2019. PMID: 30974987 Free PMC article. No abstract available.
References
-
- International Organization for Standardization. In Vitro Diagnostic Test Systems: Requirements for In Vitro Blood Glucose Monitoring Systems for Self-Testing in Managing Diabetes Mellitus. ISO15197:2015. Geneva, Switzerland: International Organization for Standardization; 2015.
-
- Food and Drug Administration: Guidance for Industry and Food and Drug Administration Staff. Self-monitoring blood glucose test systems for over-the-counter use. 2016. https://www.fda.gov/downloads/ucm380327.pdf.
-
- Klonoff DC, Parkes JL, Kovatchev BP, et al. Investigation of the accuracy of 18 marketed blood glucose monitors. Diabetes Care. 2018;41:1681-1688. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous