Microcirculatory perfusion disturbances following cardiac surgery with cardiopulmonary bypass are associated with in vitro endothelial hyperpermeability and increased angiopoietin-2 levels
- PMID: 30975180
- PMCID: PMC6460737
- DOI: 10.1186/s13054-019-2418-5
Microcirculatory perfusion disturbances following cardiac surgery with cardiopulmonary bypass are associated with in vitro endothelial hyperpermeability and increased angiopoietin-2 levels
Abstract
Background: Endothelial hyperpermeability following cardiopulmonary bypass (CPB) contributes to microcirculatory perfusion disturbances and postoperative complications after cardiac surgery. We investigated the postoperative course of renal and pulmonary endothelial barrier function and the association with microcirculatory perfusion and angiopoietin-2 levels in patients after CPB.
Methods: Clinical data, sublingual microcirculatory data, and plasma samples were collected from patients undergoing coronary artery bypass graft surgery with CPB (n = 17) before and at several time points up to 72 h after CPB. Renal and pulmonary microvascular endothelial cells were incubated with patient plasma, and in vitro endothelial barrier function was assessed using electric cell-substrate impedance sensing. Plasma levels of angiopoietin-1,-2, and soluble Tie2 were measured, and the association with in vitro endothelial barrier function and in vivo microcirculatory perfusion was determined.
Results: A plasma-induced reduction of renal and pulmonary endothelial barrier function was observed in all samples taken within the first three postoperative days (P < 0.001 for all time points vs. pre-CPB). Angiopoietin-2 and soluble Tie2 levels increased within 72 h after CPB (5.7 ± 4.4 vs. 1.7 ± 0.4 ng/ml, P < 0.0001; 16.3 ± 4.7 vs. 11.9 ± 1.9 ng/ml, P = 0.018, vs. pre-CPB), whereas angiopoietin-1 remained stable. Interestingly, reduced in vitro renal and pulmonary endothelial barrier moderately correlated with reduced in vivo microcirculatory perfusion after CPB (r = 0.47, P = 0.005; r = 0.79, P < 0.001). In addition, increased angiopoietin-2 levels moderately correlated with reduced in vitro renal and pulmonary endothelial barrier (r = - 0.46, P < 0.001; r = - 0.40, P = 0.005) and reduced in vivo microcirculatory perfusion (r = - 0.43, P = 0.01; r = - 0.41, P = 0.03).
Conclusions: CPB is associated with an impairment of in vitro endothelial barrier function that continues in the first postoperative days and correlates with reduced postoperative microcirculatory perfusion and increased circulating angiopoietin-2 levels. These results suggest that angiopoietin-2 is a biomarker for postoperative endothelial hyperpermeability, which may contribute to delayed recovery of microcirculatory perfusion after CPB.
Trial registration: NTR4212 .
Keywords: Angiopoietin-2; Capillary permeability; Cardiopulmonary bypass; Endothelium; Microcirculation.
Conflict of interest statement
Ethics approval and consent to participate
The
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

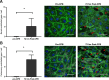
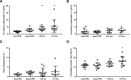

References
-
- Jongman RM, Zijlstra JG, Kok WF, van Harten AE, Mariani MA, Moser J, et al. Off-pump CABG surgery reduces systemic inflammation compared with on-pump surgery but does not change systemic endothelial responses: a prospective randomized study. Shock. 2014;42(2):121–128. doi: 10.1097/SHK.0000000000000190. - DOI - PubMed
-
- Koning NJ, Overmars MA, van den Brom CE, van Bezu J, Simon LE, Vonk ABA, et al. Endothelial hyperpermeability after cardiac surgery with cardiopulmonary bypass as assessed using an in vitro bioassay for endothelial barrier function. Br J Anaesth. 2016;116(2):223–232. doi: 10.1093/bja/aev411. - DOI - PubMed
-
- Koning NJ, de Lange F, van Meurs M, Jongman RM, Ahmed Y, Schwarte LA, et al. Reduction of vascular leakage by imatinib is associated with preserved microcirculatory perfusion and reduced renal injury in a rat model of cardiopulmonary bypass. Br J Anaesth. 2018;120(6):1165–1175. doi: 10.1016/j.bja.2017.11.095. - DOI - PubMed
-
- Dekker N.A.M., van Meurs M., van Leeuwen A.L.I., Hofland H.M., van Slyke P., Vonk A.B.A., Boer C., van den Brom C.E. Vasculotide, an angiopoietin-1 mimetic, reduces pulmonary vascular leakage and preserves microcirculatory perfusion during cardiopulmonary bypass in rats. British Journal of Anaesthesia. 2018;121(5):1041–1051. doi: 10.1016/j.bja.2018.05.049. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

