Characterising covalent warhead reactivity
- PMID: 30975501
- PMCID: PMC6538824
- DOI: 10.1016/j.bmc.2019.04.002
Characterising covalent warhead reactivity
Abstract
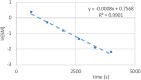
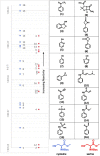
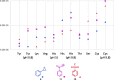
Many drugs currently used are covalent inhibitors and irreversibly inhibit their targets. Most of these were discovered through serendipity. Covalent inhibitions can have many advantages from a pharmacokinetic perspective. However, until recently most organisations have shied away from covalent compound design due to fears of non-specific inhibition of off-target proteins leading to toxicity risks. However, there has been a renewed interest in covalent modifiers as potential drugs, as it possible to get highly selective compounds. It is therefore important to know how reactive a warhead is and to be able to select the least reactive warhead possible to avoid toxicity. A robust NMR based assay was developed and used to measure the reactivity of a variety of covalent warheads against serine and cysteine - the two most common targets for covalent drugs. A selection of these warheads also had their reactivity measured against threonine, tyrosine, lysine, histidine and arginine to better understand our ability to target non-traditional residues. The reactivity was also measured at various pHs to assess what effect the environment in the active site would have on these reactions. The reactivity of a covalent modifier was found to be very dependent on the amino acid residue.
Keywords: Covalent drug; Reactivity; Warhead.
Copyright © 2019 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
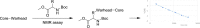
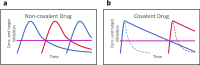

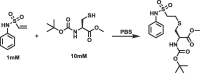

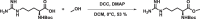
References
-
- Lonsdale R., Ward R.A. Structure-based design of targeted covalent inhibitors. Chem Soc Rev. 2018;47:3816–3830. - PubMed
-
- Tóth L., Muszbek L., Komáromi I. Mechanism of the irreversible inhibition of human cyclooxygenase-1 by aspirin as predicted by qm/mm calculations. J Mol Graph Model. 2013;40:99–109. - PubMed
-
- Yver A. Osimertinib (azd9291)-a science-driven, collaborative approach to rapid drug design and development. Ann Oncol. 2016;27:1165–1170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

