The heparin binding domain of von Willebrand factor binds to growth factors and promotes angiogenesis in wound healing
- PMID: 30975637
- PMCID: PMC6566593
- DOI: 10.1182/blood.2019000510
The heparin binding domain of von Willebrand factor binds to growth factors and promotes angiogenesis in wound healing
Abstract
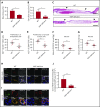
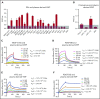
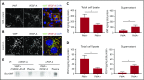
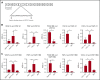
During wound healing, the distribution, availability, and signaling of growth factors (GFs) are orchestrated by their binding to extracellular matrix components in the wound microenvironment. Extracellular matrix proteins have been shown to modulate angiogenesis and promote wound healing through GF binding. The hemostatic protein von Willebrand factor (VWF) released by endothelial cells (ECs) in plasma and in the subendothelial matrix has been shown to regulate angiogenesis; this function is relevant to patients in whom VWF deficiency or dysfunction is associated with vascular malformations. Here, we show that VWF deficiency in mice causes delayed wound healing accompanied by decreased angiogenesis and decreased amounts of angiogenic GFs in the wound. We show that in vitro VWF binds to several GFs, including vascular endothelial growth factor-A (VEGF-A) isoforms and platelet-derived growth factor-BB (PDGF-BB), mainly through the heparin-binding domain (HBD) within the VWF A1 domain. VWF also binds to VEGF-A and fibroblast growth factor-2 (FGF-2) in human plasma and colocalizes with VEGF-A in ECs. Incorporation of the VWF A1 HBD into fibrin matrices enables sequestration and slow release of incorporated GFs. In vivo, VWF A1 HBD-functionalized fibrin matrices increased angiogenesis and GF retention in VWF-deficient mice. Treatment of chronic skin wounds in diabetic mice with VEGF-A165 and PDGF-BB incorporated within VWF A1 HBD-functionalized fibrin matrices accelerated wound healing, with increased angiogenesis and smooth muscle cell proliferation. Therefore, the VWF A1 HBD can function as a GF reservoir, leading to effective angiogenesis and tissue regeneration.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors’ institutions (University of Chicago and Imperial College London) filed for patent protection on aspects of the VWF HBD and its uses, and J.I., A.I., J.A.H., R.D.S., and A.M.R. are named as inventors on that patent application. The remaining authors declare no competing financial interests.
Figures


Comment in
-
von Willebrand factor promotes wound healing.Blood. 2019 Jun 13;133(24):2553-2555. doi: 10.1182/blood.2019001175. Blood. 2019. PMID: 31196875 No abstract available.
References
-
- Kawecki C, Lenting PJ, Denis CV. von Willebrand factor and inflammation. J Thromb Haemost. 2017;15(7):1285-1294. - PubMed
-
- Sadler JE, Budde U, Eikenboom JC, et al. ; Working Party on von Willebrand Disease Classification. Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor. J Thromb Haemost. 2006;4(10):2103-2114. - PubMed
-
- Barg K, Wiewiorski M, Anderson AE, et al. . Total ankle replacement in patients with von Willebrand disease: mid-term results of 18 procedures. Haemophilia. 2015;21(5):e389-e401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

