An Interaction Network of the Human SEPT9 Established by Quantitative Mass Spectrometry
- PMID: 30975701
- PMCID: PMC6553528
- DOI: 10.1534/g3.119.400197
An Interaction Network of the Human SEPT9 Established by Quantitative Mass Spectrometry
Abstract
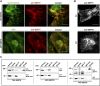
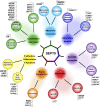
Septins regulate the organization of the actin cytoskeleton, vesicle transport and fusion, chromosome alignment and segregation, and cytokinesis in mammalian cells. SEPT9 is part of the core septin hetero-octamer in human cells which is composed of SEPT2, SEPT6, SEPT7, and SEPT9. SEPT9 has been linked to a variety of intracellular functions as well as to diseases and diverse types of cancer. A targeted high-throughput approach to systematically identify the interaction partners of SEPT9 has not yet been performed. We applied a quantitative proteomics approach to establish an interactome of SEPT9 in human fibroblast cells. Among the newly identified interaction partners were members of the myosin family and LIM domain containing proteins. Fluorescence microscopy of SEPT9 and its interaction partners provides additional evidence that SEPT9 might participate in vesicle transport from and to the plasma membrane as well as in the attachment of actin stress fibers to cellular adhesions.
Keywords: interaction map; proteomics; quantitative mass spectrometry; septins.
Copyright © 2019 Hecht et al.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
