Homoharringtonine exhibits potent anti-tumor effect and modulates DNA epigenome in acute myeloid leukemia by targeting SP1/TET1/5hmC
- PMID: 30975912
- PMCID: PMC6939512
- DOI: 10.3324/haematol.2018.208835
Homoharringtonine exhibits potent anti-tumor effect and modulates DNA epigenome in acute myeloid leukemia by targeting SP1/TET1/5hmC
Abstract
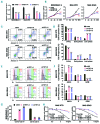
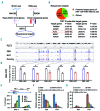
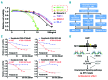
Homoharringtonine, a plant alkaloid, has been reported to suppress protein synthesis and has been approved by the US Food and Drug Administration for the treatment of chronic myeloid leukemia. Here we show that in acute myeloid leukemia (AML), homoharringtonine potently inhibits cell growth/viability and induces cell cycle arrest and apoptosis, significantly inhibits disease progression in vivo, and substantially prolongs survival of mice bearing murine or human AML. Strikingly, homoharringtonine treatment dramatically decreases global DNA 5-hydroxymethylcytosine abundance through targeting the SP1/TET1 axis, and TET1 depletion mimics homoharringtonine's therapeutic effects in AML. Our further 5hmC-seq and RNA-seq analyses, followed by a series of validation and functional studies, suggest that FLT3 is a critical down-stream target of homoharringtonine/SP1/TET1/5hmC signaling, and suppression of FLT3 and its downstream targets (e.g. MYC) contributes to the high sensitivity of FLT3-mutated AML cells to homoharringtonine. Collectively, our studies uncover a previously unappreciated DNA epigenome-related mechanism underlying the potent antileukemic effect of homoharringtonine, which involves suppression of the SP1/TET1/5hmC/FLT3/MYC signaling pathways in AML. Our work also highlights the particular promise of clinical application of homoharringtonine to treat human AML with FLT3 mutations, which accounts for more than 30% of total cases of AML.
Copyright© 2020 Ferrata Storti Foundation.
Figures




Comment in
-
A new kid on the block for acute myeloid leukemia treatment? Homoharringtonine interferes with key pathways in acute myeloid leukemia cells.Haematologica. 2020 Jan;105(1):7-9. doi: 10.3324/haematol.2019.234880. Haematologica. 2020. PMID: 31894095 Free PMC article. No abstract available.
References
-
- Powell RG, Weisleder D, Smith CR., Jr Antitumor alkaloids for Cephalataxus harringtonia: structure and activity. J Pharm Sci. 1972;61(8):1227–1230. - PubMed
-
- Nazha A, Kantarjian H, Cortes J, Quintas-Cardama A. Omacetaxine mepesuccinate (synribo) - newly launched in chronic myeloid leukemia. Expert Opin Pharmacother. 2013;14(14):1977–1986. - PubMed
-
- Cephalotaxine esters in the treatment of acute leukemia. A preliminary clinical assessment. Chin Med J (Engl). 1976; 2(4):263–272. - PubMed
-
- Jin J, Jiang DZ, Mai WY, et al. Homoharringtonine in combination with cytarabine and aclarubicin resulted in high complete remission rate after the first induction therapy in patients with de novo acute myeloid leukemia. Leukemia. 2006; 20(8):1361–1367. - PubMed
-
- Jin J, Wang JX, Chen FF, et al. Homoharringtonine-based induction regimens for patients with de-novo acute myeloid leukaemia: a multicentre, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2013;14(7):599–608. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

