The route of the visual receptor rhodopsin along the cilium
- PMID: 30975916
- PMCID: PMC6550008
- DOI: 10.1242/jcs.229526
The route of the visual receptor rhodopsin along the cilium
Abstract
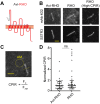
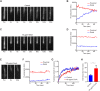
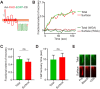
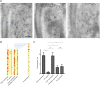
The photoreceptor outer segment is the most elaborate primary cilium, containing large amounts of rhodopsin (RHO) in disk membranes that grow from a connecting cilium. The movement of RHO along the connecting cilium precedes formation of the disk membranes. However, the route that RHO takes has not been clearly determined; some reports suggest that it follows an intracellular, vesicular route along the axoneme, possibly as an adaptation for the high load of delivery or the morphogenesis of the disk endomembranes. We addressed this question by studying RHO in cilia of IMCD3 cells and mouse rod photoreceptors. In IMCD3 cilia, fluorescence recovery after photobleaching (FRAP) experiments with fluorescently tagged RHO supported the idea of RHO motility in the ciliary plasma membrane and was inconsistent with the hypothesis of RHO motility within the lumen of the cilium. In rod photoreceptors, FRAP of RHO-EGFP was altered by externally applied lectin, supporting the idea of plasmalemmal RHO dynamics. Quantitative immunoelectron microscopy corroborated our live-cell conclusions, as RHO was found to be distributed along the plasma membrane of the connecting cilium, with negligible labeling within the axoneme. Taken together, the present findings demonstrate RHO trafficking entirely via the ciliary plasma membrane.This article has an associated First Person interview with the first author of the paper.
Keywords: Cilium; Photoreceptor; Rhodopsin; Trafficking.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

References
-
- Abd-El-Barr M. M., Sykoudis K., Andrabi S., Eichers E. R., Pennesi M. E., Tan P. L., Wilson J. H., Katsanis N., Lupski J. R. and Wu S. M. (2007). Impaired photoreceptor protein transport and synaptic transmission in a mouse model of Bardet-Biedl syndrome. Vision Res. 47, 3394-3407. 10.1016/j.visres.2007.09.016 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

