Metabolic regulation of cell growth and proliferation
- PMID: 30976106
- PMCID: PMC6592760
- DOI: 10.1038/s41580-019-0123-5
Metabolic regulation of cell growth and proliferation
Abstract
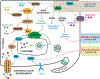
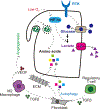
Cellular metabolism is at the foundation of all biological activities. The catabolic processes that support cellular bioenergetics and survival have been well studied. By contrast, how cells alter their metabolism to support anabolic biomass accumulation is less well understood. During the commitment to cell proliferation, extensive metabolic rewiring must occur in order for cells to acquire sufficient nutrients such as glucose, amino acids, lipids and nucleotides, which are necessary to support cell growth and to deal with the redox challenges that arise from the increased metabolic activity associated with anabolic processes. Defining the mechanisms of this metabolic adaptation for cell growth and proliferation is now a major focus of research. Understanding the principles that guide anabolic metabolism may ultimately enhance ways to treat diseases that involve deregulated cell growth and proliferation, such as cancer.
Conflict of interest statement
Competing interests
C.B.T. is a founder of Agios Pharmaceuticals and a member of its scientific advisory board. He is also a former member of the Board of Directors and stockholder of Merck and Charles River Laboratories. He has patents related to cellular metabolism.
Figures




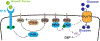
References
-
- Rheinwald JG & Green H Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature 265, 421–424 (1977). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

