Protease-activated receptors (PARs): mechanisms of action and potential therapeutic modulators in PAR-driven inflammatory diseases
- PMID: 30976204
- PMCID: PMC6440139
- DOI: 10.1186/s12959-019-0194-8
Protease-activated receptors (PARs): mechanisms of action and potential therapeutic modulators in PAR-driven inflammatory diseases
Erratum in
-
Correction to: protease-activated receptors (PARs): mechanisms of action and potential therapeutic modulators in PAR-driven inflammatory diseases.Thromb J. 2019 Nov 6;17:22. doi: 10.1186/s12959-019-0212-x. eCollection 2019. Thromb J. 2019. PMID: 31708692 Free PMC article.
Abstract
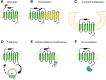
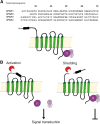
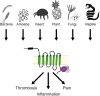
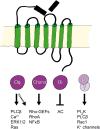
Inflammatory diseases have become increasingly prevalent with industrialization. To address this, numerous anti-inflammatory agents and molecular targets have been considered in clinical trials. Among molecular targets, protease-activated receptors (PARs) are abundantly recognized for their roles in the development of chronic inflammatory diseases. In particular, several inflammatory effects are directly mediated by the sensing of proteolytic activity by PARs. PARs belong to the seven transmembrane domain G protein-coupled receptor family, but are unique in their lack of physiologically soluble ligands. In contrast with classical receptors, PARs are activated by N-terminal proteolytic cleavage. Upon removal of specific N-terminal peptides, the resulting N-termini serve as tethered activation ligands that interact with the extracellular loop 2 domain and initiate receptor signaling. In the classical pathway, activated receptors mediate signaling by recruiting G proteins. However, activation of PARs alternatively lead to the transactivation of and signaling through receptors such as co-localized PARs, ion channels, and toll-like receptors. In this review we consider PARs and their modulators as potential therapeutic agents, and summarize the current understanding of PAR functions from clinical and in vitro studies of PAR-related inflammation.
Conflict of interest statement
Not applicable.Not applicable.The authors declare that they have no competing interests.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
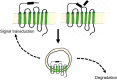
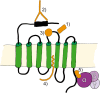
References
-
- Bahou WF, Nierman WC, Durkin AS, Potter CL, Demetrick DJ. Chromosomal assignment of the human thrombin receptor gene: localization to region q13 of chromosome 5. Blood. 1993;82:1532–1537. - PubMed
-
- Schmidt VA, Nierman WC, Maglott DR, Cupit LD, Moskowitz KA, Wainer JA, Bahou WF. The human proteinase-activated receptor-3 (PAR-3) gene. Identification within a par gene cluster and characterization in vascular endothelial cells and platelets. J Biol Chem. 1998;273:15061–15068. doi: 10.1074/jbc.273.24.15061. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

