Surgery in the age of biologics
- PMID: 30976420
- PMCID: PMC6454839
- DOI: 10.1093/gastro/goz004
Surgery in the age of biologics
Abstract
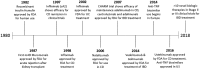
Since the introduction of the first anti-tumor necrosis factor antibodies in the late 1990s, biologic therapy has revolutionized the medical treatment of patients with inflammatory bowel disease (IBD). Nevertheless, surgery continues to play a significant role in treating IBD patients. Rates of intestinal resection in patients with Crohn's disease or colectomy in ulcerative colitis are reducing but not substantially over the long term. An increasing variety of biologic medications are now available to treat IBD patients in various clinical situations. Consequently, a number of questions persist about how biologic medications affect the need for surgery and overall course in IBD patients. Given the trend for earlier and more frequent use of biologic medications in IBD patients, a working knowledge of the effects of these medications on surgical decision-making and outcomes is essential for the practicing colorectal surgeon and gastroenterologist. This review seeks to summarize the relevant literature surrounding biologic use and IBD surgery with a focus on the effect of biologics on the frequency, type and complications of surgery in this 'age of biologics'.
Keywords: Biologics; inflammatory bowel disease; review; surgery.
Figures
References
-
- Gunnarsson C, Chen J, Rizzo JA.. Direct health care insurer and out-of-pocket expenditures of inflammatory bowel disease: evidence from a US national survey. Dig Dis Sci 2012;57:3080–91. - PubMed
-
- Mao R, Hu PJ.. The future of IBD therapy: where are we and where should we go next? Dig Dis 2016;34:175–9. - PubMed
-
- Langholz E, Munkholm P, Davidsen M. et al. Course of ulcerative colitis: analysis of changes in disease activity over years. Gastroenterology 1994;107:3–11. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources