Characterizing the selectivity of ER α-glucosidase inhibitors
- PMID: 30976784
- PMCID: PMC6583763
- DOI: 10.1093/glycob/cwz029
Characterizing the selectivity of ER α-glucosidase inhibitors
Erratum in
-
Correction to: Characterizing the selectivity of ER α-glucosidase inhibitors.Glycobiology. 2023 Jun 21;33(6):525. doi: 10.1093/glycob/cwad017. Glycobiology. 2023. PMID: 36975755 Free PMC article. No abstract available.
Abstract
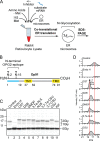
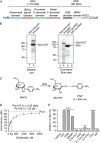
The endoplasmic reticulum (ER) contains both α-glucosidases and α-mannosidases which process the N-linked oligosaccharides of newly synthesized glycoproteins and thereby facilitate polypeptide folding and glycoprotein quality control. By acting as structural mimetics, iminosugars can selectively inhibit these ER localized α-glycosidases, preventing N-glycan trimming and providing a molecular basis for their therapeutic applications. In this study, we investigate the effects of a panel of nine iminosugars on the actions of ER luminal α-glucosidase I and α-glucosidase II. Using ER microsomes to recapitulate authentic protein N-glycosylation and oligosaccharide processing, we identify five iminosugars that selectively inhibit N-glycan trimming. Comparison of their inhibitory activities in ER microsomes against their effects on purified ER α-glucosidase II, suggests that 3,7a-diepi-alexine acts as a selective inhibitor of ER α-glucosidase I. The other active iminosugars all inhibit α-glucosidase II and, having identified 1,4-dideoxy-1,4-imino-D-arabinitol (DAB) as the most effective of these compounds, we use in silico modeling to understand the molecular basis for this enhanced activity. Taken together, our work identifies the C-3 substituted pyrrolizidines casuarine and 3,7a-diepi-alexine as promising "second-generation" iminosugar inhibitors.
Keywords: N-linked glycosylation; endoplasmic reticulum; glucose trimming; iminosugar inhibitors.
© The Author(s) 2019. Published by Oxford University Press.
Figures




References
-
- Asano N. 2008. Glycosidase-inhibiting alkaloids: isolation, structure and application In: Fattorusso E, Taglialatela-Scafati O, editors. Modern Alkaloids: Structure, Isolation, Synthesis and Biology. Weinheim: Wiley-VCH Verlag; p. 111–138.
-
- Asano N, Nash RJ, Molyneux RJ, Fleet GWJ. 2000. Sugar-mimic glycosidase inhibitors: natural occurrence, biological activity and prospects for therapeutic application. Tetrahedron: Asymmetry. 11:1645–1680.
-
- Asano N, Oseki K, Kizu H, Matsui K. 1994. Nitrogen-in-the-ring pyranoses and furanoses: structural basis of inhibition of mammalian glycosidases. J Med Chem. 37:3701–3706. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

