Uninterrupted edoxaban vs. vitamin K antagonists for ablation of atrial fibrillation: the ELIMINATE-AF trial
- PMID: 30976787
- PMCID: PMC6754569
- DOI: 10.1093/eurheartj/ehz190
Uninterrupted edoxaban vs. vitamin K antagonists for ablation of atrial fibrillation: the ELIMINATE-AF trial
Erratum in
-
Corrigendum to: Uninterrupted edoxaban vs. vitamin K antagonists for ablation of atrial fibrillation: the ELIMINATE-AF trial.Eur Heart J. 2019 Sep 21;40(36):3043. doi: 10.1093/eurheartj/ehz630. Eur Heart J. 2019. PMID: 31504419 Free PMC article. No abstract available.
Abstract
Aims: Edoxaban is a direct factor Xa inhibitor approved for stroke prevention in atrial fibrillation (AF). Uninterrupted edoxaban therapy in patients undergoing AF ablation has not been tested.
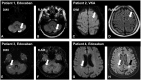
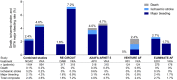
Methods and results: The ELIMINATE-AF trial, a multinational, multicentre, randomized, open-label, parallel-group study, was conducted to assess the safety and efficacy of once-daily edoxaban 60 mg (30 mg in patients indicated for dose reduction) vs. vitamin K antagonists (VKAs) in AF patients undergoing catheter ablation. Patients were randomized 2:1 to edoxaban vs. VKA. The primary endpoint (per-protocol population) was time to first occurrence of all-cause death, stroke, or International Society of Thrombosis and Haemostasis-defined major bleeding during the period from the end of the ablation procedure to end of treatment (90 days). Overall, 632 patients were enrolled, 614 randomized, and 553 received study drug and underwent ablation; 177 subjects underwent brain magnetic resonance imaging to assess silent cerebral infarcts. The primary endpoint (only major bleeds occurred) was observed in 0.3% (1 patient) on edoxaban and 2.0% (2 patients) on VKA [hazard ratio (95% confidence interval): 0.16 (0.02-1.73)]. In the ablation population (modified intent-to-treat population including patients with ablation), the primary endpoint was observed in 2.7% of edoxaban (N = 10) and 1.7% of VKA patients (N = 3) between start of ablation and end of treatment. There were one ischaemic and one haemorrhagic stroke, both in patients on edoxaban. Cerebral microemboli were detected in 13.8% (16) patients who received edoxaban and 9.6% (5) patients in the VKA group (nominal P = 0.62).
Conclusion: Uninterrupted edoxaban therapy represents an alternative to uninterrupted VKA treatment in patients undergoing AF ablation.
Keywords: Ablation; Anticoagulation; Atrial fibrillation; Bleeding events; Stroke.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures
Comment in
-
Continuous anticoagulation with catheter ablation: answers and questions.Eur Heart J. 2019 Sep 21;40(36):3022-3025. doi: 10.1093/eurheartj/ehz322. Eur Heart J. 2019. PMID: 31168627 No abstract available.
References
-
- Calkins H, Hindricks G, Cappato R, Kim Y-H, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, Chen P-S, Chen S-A, Chung MK, Nielsen JC, Curtis AB, Davies DW, Day JD, d’Avila A, de Groot NMS(N), Di Biase L, Duytschaever M, Edgerton JR, Ellenbogen KA, Ellinor PT, Ernst S, Fenelon G, Gerstenfeld EP, Haines DE, Haissaguerre M, Helm RH, Hylek E, Jackman WM, Jalife J, Kalman JM, Kautzner J, Kottkamp H, Kuck KH, Kumagai K, Lee R, Lewalter T, Lindsay BD, Macle L, Mansour M, Marchlinski FE, Michaud GF, Nakagawa H, Natale A, Nattel S, Okumura K, Packer D, Pokushalov E, Reynolds MR, Sanders P, Scanavacca M, Schilling R, Tondo C, Tsao H-M, Verma A, Wilber DJ, Yamane T.. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017;14:e275–e444. - PMC - PubMed
-
- January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; ACC AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:e199–e267. - PMC - PubMed
-
- Di Biase L, Burkhardt JD, Santangeli P, Mohanty P, Sanchez JE, Horton R, Gallinghouse GJ, Themistoclakis S, Rossillo A, Lakkireddy D, Reddy M, Hao S, Hongo R, Beheiry S, Zagrodzky J, Rong B, Mohanty S, Elayi CS, Forleo G, Pelargonio G, Narducci ML, Dello Russo A, Casella M, Fassini G, Tondo C, Schweikert RA, Natale A.. Periprocedural stroke and bleeding complications in patients undergoing catheter ablation of atrial fibrillation with different anticoagulation management: results from the Role of Coumadin in Preventing Thromboembolism in Atrial Fibrillation (AF) Patients Undergoing Catheter Ablation (COMPARE) randomized trial. Circulation 2014;129:2638–2644. - PubMed
-
- Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM.. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–962. - PubMed
-
- Calkins H, Willems S, Gerstenfeld EP, Verma A, Schilling R, Hohnloser SH, Okumura K, Serota H, Nordaby M, Guiver K, Biss B, Brouwer MA, Grimaldi M, Circuit Investigators RE.. Uninterrupted dabigatran versus warfarin for ablation in atrial fibrillation. N Engl J Med 2017;376:1627–1636. - PubMed

