Arabidopsis glucosinolate storage cells transform into phloem fibres at late stages of development
- PMID: 30976798
- PMCID: PMC6698705
- DOI: 10.1093/jxb/erz176
Arabidopsis glucosinolate storage cells transform into phloem fibres at late stages of development
Abstract
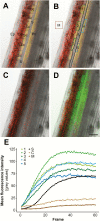
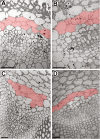
The phloem cap of Arabidopsis thaliana accumulates glucosinolates that yield toxic catabolites upon damage-induced hydrolysis. These defence compounds are stored in high concentrations in millimetre long S-cells. At early stages of development, S-cells initiate a process indicative of programmed cell death. How these cells are maintained in a highly turgescent state following this process is currently unknown. Here, we show that S-cells undergo substantial morphological changes during early differentiation. Vacuolar collapse and rapid clearance of the cytoplasm did not occur until senescence. Instead, smooth endoplasmic reticulum, Golgi bodies, vacuoles, and undifferentiated plastids were observed. Lack of chloroplasts indicates that S-cells depend on metabolite supply from neighbouring cells. Interestingly, TEM revealed numerous plasmodesmata between S-cells and neighbouring cells. Photoactivation of a symplasmic tracer showed coupling with neighbouring cells that are involved in glucosinolate synthesis. Hence, symplasmic transport might contribute to glucosinolate storage in S-cells. To investigate the fate of S-cells, we traced them in flower stalks from the earliest detectable stages to senescence. At late stages, S-cells were shown to deposit thick secondary cell walls and transform into phloem fibres. Thus, phloem fibres in the herbaceous plant Arabidopsis pass a pronounced phase of chemical defence during early stages of development.
Keywords: Glucosinolates; S-cell; phloem cap; phloem fibre; photoactivation; plasmodesmata.
© The Author(s) 2019. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures




References
-
- Ageeva MV, Petrovská B, Kieft H, Sal’nikov VV, Snegireva AV, van Dam JE, van Veenendaal WL, Emons AM, Gorshkova TA, van Lammeren AA. 2005. Intrusive growth of flax phloem fibers is of intercalary type. Planta 222, 565–574. - PubMed
-
- Altamura MM, Possenti M, Matteucci A, Baima S, Ruberti I, Morelli G. 2001. Development of the vascular system in the inflorescence stem of Arabidopsis. New Phytologist 151, 381–389.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

