G-protein coupled receptors and ligands that organize humoral immune responses
- PMID: 30977196
- PMCID: PMC6464390
- DOI: 10.1111/imr.12743
G-protein coupled receptors and ligands that organize humoral immune responses
Abstract
B-cell responses are dynamic processes that depend on multiple types of interactions. Rare antigen-specific B cells must encounter antigen and specialized systems are needed-unique to each lymphoid tissue type-to ensure this happens efficiently. Lymphoid tissue barrier cells act to ensure that pathogens, while being permitted entry for B-cell recognition, are blocked from replication or dissemination. T follicular helper (Tfh) cells often need to be primed by dendritic cells before supporting B-cell responses. For most responses, antigen-specific helper T cells and B cells need to interact, first to initiate clonal expansion and the plasmablast response, and later to support the germinal center (GC) response. Newly formed plasma cells need to travel to supportive niches. GC B cells must become confined to the follicle center, organize into dark and light zones, and interact with Tfh cells. Memory B cells need to be positioned for rapid responses following reinfection. Each of these events requires the actions of multiple G-protein coupled receptors (GPCRs) and their ligands, including chemokines and lipid mediators. This review will focus on the guidance cue code underlying B-cell immunity, with an emphasis on findings from our laboratory and on newer advances in related areas. We will discuss our recent identification of geranylgeranyl-glutathione as a ligand for P2RY8. Our goal is to provide the reader with a focused knowledge about the GPCRs guiding B-cell responses and how they might be therapeutic targets, while also providing examples of how multiple types of GPCRs can cooperate or act iteratively to control cell behavior.
Keywords: CXCR4; CXCR5; EBI2; P2RY8; S1PR2; chemoattractant; migration-inhibition.
© 2019 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures

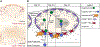

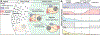
References
-
- Cyster JG, Ansel KM, Reif K, et al. Follicular stromal cells and lymphocyte homing to follicles. Immunol Rev.2000;176:181–193. - PubMed
-
- Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol.2014;32:659–702. - PubMed
-
- Schulz O, Hammerschmidt SI, Moschovakis GL, Forster R. Chemokines and Chemokine Receptors in Lymphoid Tissue Dynamics. Annu Rev Immunol.2016;34:203–242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

