Defects in intervertebral disc and spine during development, degeneration, and pain: New research directions for disc regeneration and therapy
- PMID: 30977275
- PMCID: PMC6565447
- DOI: 10.1002/wdev.343
Defects in intervertebral disc and spine during development, degeneration, and pain: New research directions for disc regeneration and therapy
Abstract
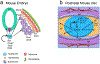
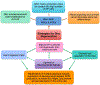
Intervertebral discs are cartilaginous joints present between vertebrae. The centers of the intervertebral discs consist of a gelatinous nucleus pulposus derived from the embryonic notochord. With age or injury, intervertebral discs may degenerate, causing neurological symptoms including back pain, which affects millions of people worldwide. Back pain is a multifactorial disorder, and disc degeneration is one of the primary contributing factors. Recent studies in mice have identified the key molecules involved in the formation of intervertebral discs. Several of these key molecules including sonic hedgehog and Brachyury are not only expressed by notochord during development, but are also expressed by neonatal mouse nucleus pulposus cells, and are crucial for postnatal disc maintenance. These findings suggest that intrinsic signals in each disc may maintain the nucleus pulposus microenvironment. However, since expression of these developmental signals declines with age and degeneration, disc degeneration may be related to the loss of these intrinsic signals. In addition, findings from mouse and other mammalian models have identified similarities between the patterning capabilities of the embryonic notochord and young nucleus pulposus cells, suggesting that mouse is a suitable model system to understand disc development and aging. Future research aimed at understanding the upstream regulators of these developmental signals and the modes by which they regulate disc growth and maintenance will likely provide mechanistic insights into disc growth and aging. Further, such findings will likely provide insights relevant to the development of effective therapies for treatment of back pain and reversing the disc degenerative process. This article is categorized under: Birth Defects > Organ Anomalies Vertebrate Organogenesis > Musculoskeletal and Vascular Adult Stem Cells, Tissue Renewal, and Regeneration > Regeneration Adult Stem Cells, Tissue Renewal, and Regeneration > Stem Cells and Aging.
Keywords: Brachyury; Shh; disc degeneration; lower back pain; regeneration.
© 2019 Wiley Periodicals, Inc.
Conflict of interest statement
No conflicts to declare
Figures




References
-
- Adams DS, Keller R, & Koehl MA (1990). The mechanics of notochord elongation, straightening and stiffening in the embryo of Xenopus laevis. Development, 110(1), 115–130. - PubMed
-
- Arnoldi CC, Brodsky AE, Cauchoix J, Crock HV, Dommisse GF, Edgar MA, . . . Wiltse LL (1976). Lumbar spinal stenosis and nerve root entrapment syndromes. Definition and classification. Clin Orthop Relat Res(115), 4–5. - PubMed
-
- Bach FC, de Vries SA, Riemers FM, Boere J, van Heel FW, van Doeselaar M, . . . Tryfonidou MA (2016). Soluble and pelletable factors in porcine, canine and human notochordal cell-conditioned medium: implications for IVD regeneration. Eur Cell Mater, 32, 163–180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

