Regulatory principles governing the maternal-to-zygotic transition: insights from Drosophila melanogaster
- PMID: 30977698
- PMCID: PMC6303782
- DOI: 10.1098/rsob.180183
Regulatory principles governing the maternal-to-zygotic transition: insights from Drosophila melanogaster
Abstract
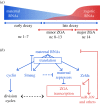
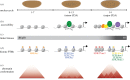
The onset of metazoan development requires that two terminally differentiated germ cells, a sperm and an oocyte, become reprogrammed to the totipotent embryo, which can subsequently give rise to all the cell types of the adult organism. In nearly all animals, maternal gene products regulate the initial events of embryogenesis while the zygotic genome remains transcriptionally silent. Developmental control is then passed from mother to zygote through a process known as the maternal-to-zygotic transition (MZT). The MZT comprises an intimately connected set of molecular events that mediate degradation of maternally deposited mRNAs and transcriptional activation of the zygotic genome. This essential developmental transition is conserved among metazoans but is perhaps best understood in the fruit fly, Drosophila melanogaster. In this article, we will review our understanding of the events that drive the MZT in Drosophila embryos and highlight parallel mechanisms driving this transition in other animals.
Keywords: Drosophila; Zelda; cellular reprogramming; embryogenesis; maternal-to-zygotic transition; zygotic genome activation.
Conflict of interest statement
We declare we have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

