Mitochondrial angiotensin receptors and cardioprotective pathways
- PMID: 30978131
- PMCID: PMC6620675
- DOI: 10.1152/ajpheart.00772.2018
Mitochondrial angiotensin receptors and cardioprotective pathways
Abstract
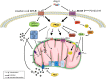
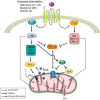
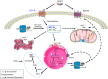
A growing body of data provides strong evidence that intracellular angiotensin II (ANG II) plays an important role in mammalian cell function and is involved in the pathogenesis of human diseases such as hypertension, diabetes, inflammation, fibrosis, arrhythmias, and kidney disease, among others. Recent studies also suggest that intracellular ANG II exerts protective effects in cells during high extracellular levels of the hormone or during chronic stimulation of the local tissue renin-angiotensin system (RAS). Notably, the intracellular RAS (iRAS) described in neurons, fibroblasts, renal cells, and cardiomyocytes provided new insights into regulatory mechanisms mediated by intracellular ANG II type 1 (AT1Rs) and 2 (AT2Rs) receptors, particularly, in mitochondria and nucleus. For instance, ANG II through nuclear AT1Rs promotes protective mechanisms by stimulating the AT2R signaling cascade, which involves mitochondrial AT2Rs and Mas receptors. The stimulation of nuclear ANG II receptors enhances mitochondrial biogenesis through peroxisome proliferator-activated receptor-γ coactivator-1α and increases sirtuins activity, thus protecting the cell against oxidative stress. Recent studies in ANG II-induced preconditioning suggest that plasma membrane AT2R stimulation exerts protective effects against cardiac ischemia-reperfusion by modulating mitochondrial AT1R and AT2R signaling. These studies indicate that iRAS promotes the protection of cells through nuclear AT1R signaling, which, in turn, promotes AT2R-dependent processes in mitochondria. Thus, despite abundant data on the deleterious effects of intracellular ANG II, a growing body of studies also supports a protective role for iRAS that could be of relevance to developing new therapeutic strategies. This review summarizes and discusses previous studies on the role of iRAS, particularly emphasizing the protective and counterbalancing actions of iRAS, mitochondrial ANG II receptors, and their implications for organ protection.
Keywords: angiotensin II receptors; cardioprotection; heart; intracellular renin-angiotensin system; mitochondria.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
References
-
- Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, Smith BJ, Burks TN, Cohn RD, Fedarko NS, Carey RM, O’Rourke B, Walston JD. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci USA 108: 14849–14854, 2011. doi: 10.1073/pnas.1101507108. - DOI - PMC - PubMed
-
- Arnaud C, Joyeux-Faure M, Bottari S, Godin-Ribuot D, Ribuot C. New insight into the signalling pathways of heat stress-induced myocardial preconditioning: protein kinase Cepsilon translocation and heat shock protein 27 phosphorylation. Clin Exp Pharmacol Physiol 31: 129–133, 2004. doi: 10.1111/j.1440-1681.2004.03966.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

