Stressed: The Unfolded Protein Response in T Cell Development, Activation, and Function
- PMID: 30978945
- PMCID: PMC6479341
- DOI: 10.3390/ijms20071792
Stressed: The Unfolded Protein Response in T Cell Development, Activation, and Function
Abstract
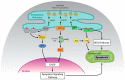
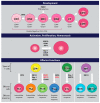
The unfolded protein response (UPR) is a highly conserved pathway that allows cells to respond to stress in the endoplasmic reticulum caused by an accumulation of misfolded and unfolded protein. This is of great importance to secretory cells because, in order for proteins to traffic from the endoplasmic reticulum (ER), they need to be folded appropriately. While a wealth of literature has implicated UPR in immune responses, less attention has been given to the role of UPR in T cell development and function. This review discusses the importance of UPR in T cell development, homeostasis, activation, and effector functions. We also speculate about how UPR may be manipulated in T cells to ameliorate pathologies.
Keywords: ER stress; T cells; UPR; protein folding.
Conflict of interest statement
The authors declare they have no conflicts of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

