Immunization with Components of the Viral Fusion Apparatus Elicits Antibodies That Neutralize Epstein-Barr Virus in B Cells and Epithelial Cells
- PMID: 30979688
- PMCID: PMC6660903
- DOI: 10.1016/j.immuni.2019.03.010
Immunization with Components of the Viral Fusion Apparatus Elicits Antibodies That Neutralize Epstein-Barr Virus in B Cells and Epithelial Cells
Abstract
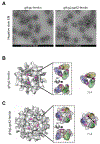
Epstein-Barr virus (EBV) causes infectious mononucleosis and is associated with epithelial-cell cancers and B cell lymphomas. An effective EBV vaccine is not available. We found that antibodies to the EBV glycoprotein gH/gL complex were the principal components in human plasma that neutralized infection of epithelial cells and that antibodies to gH/gL and gp42 contributed to B cell neutralization. Immunization of mice and nonhuman primates with nanoparticle vaccines that displayed components of the viral-fusion machinery EBV gH/gL or gH/gL/gp42 elicited antibodies that potently neutralized both epithelial-cell and B cell infection. Immune serum from nonhuman primates inhibited EBV-glycoprotein-mediated fusion of epithelial cells and B cells and targeted an epitope critical for virus-cell fusion. Therefore, unlike the leading EBV gp350 vaccine candidate, which only protects B cells from infection, these EBV nanoparticle vaccines elicit antibodies that inhibit the virus-fusion apparatus and provide cell-type-independent protection from virus infection.
Keywords: B cell lymphoma; Epstein-Barr virus; infectious mononucleosis; nanoparticle; vaccine; virus fusion.
Published by Elsevier Inc.
Conflict of interest statement
DECLARATION OF INTERESTS
M.K., W.B., G.J.N. and J.I.C. are named as inventors on a patent application describing the data presented in this paper, which have been filed by the National Institutes of Health. G.N. is an employee of Sanofi.
Figures

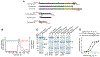

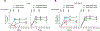


References
-
- Bachmann MF, Rohrer UH, Kündig TM, Bürki K, Hengartner H, and Zinkernagel RM (1993). The influence of antigen organization on B cell responsiveness. Science. 262, 1448–1451. - PubMed
-
- Blasco R, and Moss B (1995). Selection of recombinant vaccinia viruses on the basis of plaque formation. Gene 158, 157–162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

