International collaborative actions and transparency to understand, diagnose, and develop therapies for rare diseases
- PMID: 30979709
- PMCID: PMC6505568
- DOI: 10.15252/emmm.201910486
International collaborative actions and transparency to understand, diagnose, and develop therapies for rare diseases
Abstract
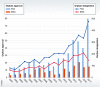
Rare diseases, which affect over 350 million people worldwide and frequently go undiagnosed or misdiagnosed for years, suffer from sparse and dispersed medical knowledge leading to even rarer approved and effective therapeutic options for patients. A vast, unmet need for research and investment to advance diagnostic capabilities and therapeutic development must be confronted, despite the myriad of challenges faced. Several fundamental shifts are changing the landscape of rare diseases research and development, particularly with the application and extension of results to common diseases and the advancement of personalized medicine initiatives. Collaborative strategies that pool resources and knowledge are vital, including team science, research networks, novel funding models, shared knowledge platforms, and innovative regulatory frameworks. Importantly, patients are also increasingly involved as research partners and funders, pushing for open science and transparency, and breaking down data silos and geographical borders, often enabled by online platforms accessible from across the globe. The International Rare Diseases Research Consortium (
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- EMA (2018) European Medicines Agency (EMA): orphan medicines figures 2000–2017. https://www.ema.europa.eu/documents/other/orphan-medicines-figures-2000-.... Accessed 20 November 2018
-
- FDA (2018) US Food and Drug Administration (FDA): orphan drug designations and approvals. https://www.accessdata.fda.gov/scripts/opdlisting/oopd/. Accessed 20 November 2018
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

