Inhibitory Units: An Organizing Nidus for Feature-Selective SubNetworks in Area V1
- PMID: 30979814
- PMCID: PMC6670246
- DOI: 10.1523/JNEUROSCI.2275-18.2019
Inhibitory Units: An Organizing Nidus for Feature-Selective SubNetworks in Area V1
Abstract
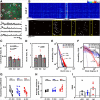
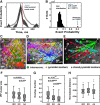
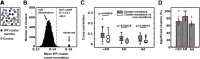
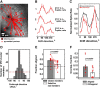
Neuronal circuits often display small-world network architecture characterized by neuronal cliques of dense local connectivity communicating with each other through a limited number of cells that participate in multiple cliques. The principles by which such cliques organize to encode information remain poorly understood. Similarly tuned pyramidal cells that preferentially target each other may form multicellular encoding units performing distinct computational tasks. The existence of such units can reflect upon both spontaneous and stimulus-driven population events.We applied two-photon calcium imaging to study spontaneous population bursts in layer 2/3 of area V1 in male C57BL/6 mice. To identify potential small-world cliques, we searched for pyramidal cells whose calcium events had a consistent temporal relationship with the events of local inhibitory interneurons. This was guided by the intuition that groups of neurons whose synchronous firing represents a temporally coherent computational unit should be inhibited together. Pyramidal members of these interneuron-centered clusters on average displayed stronger functional connectivity between each other than with nonmember pyramidal neurons. The structure of the clusters evolved during postnatal development: cluster size and overlap between clusters decreased with developmental maturation. Pyramidal neurons in a cluster showed higher than chance tuning function similarity between each other and with the linked interneuron. Thus, spontaneous population events in V1 are shaped by small-world subnetworks of pyramidal neurons that share functional properties and work as a coherent unit with a local interneuron. These interneuron-pyramidal cell partnerships may represent a fundamental neocortical unit of computation at the population level.SIGNIFICANCE STATEMENT Neuronal circuit in layer 2/3 of mouse area V1 possesses small-world network architecture, where cliques of densely interconnected neurons ("small worlds") communicate via restricted number of hub cells. We show that: (1) in mouse V1 individual small-world cliques preferably incorporate pyramidal neurons with similar visual feature tuning, and (2) ongoing population activity of such pyramidal neuron clique is temporally linked to the activity of the local interneuron sharing its feature tuning with the clique members. Functional grouping of similarly tuned interneurons and pyramidal cells into cliques may ensure that ensembles of functionally alike pyramidal cells recruited during perceptual tasks and spontaneous activity are also turned off together as a unit, with interneurons serving as organizers of linked pyramidal ensemble activity.
Keywords: interneuron-pyramidal cell clusters; modularity; multineuronal bursts; neuronal ensembles; small world; spontaneous activity.
Copyright © 2019 the authors.
Figures

References
-
- Abramoff MD, Magalhaes PJ, Ram SJ (2004) Image processing with ImageJ. Biophotonics Int 11:36–42.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
