Evaluation of seizure treatment in anti-LGI1, anti-NMDAR, and anti-GABABR encephalitis
- PMID: 30979857
- PMCID: PMC6537134
- DOI: 10.1212/WNL.0000000000007475
Evaluation of seizure treatment in anti-LGI1, anti-NMDAR, and anti-GABABR encephalitis
Abstract
Objective: This nationwide cohort study evaluates seizure responses to immunotherapy and antiepileptic drugs (AEDs) in patients with anti-leucine-rich glioma-inactivated 1 (LGI1), anti-NMDA receptor (NMDAR), and anti-gamma-aminobutyric-acid B receptor (GABABR) encephalitis.
Methods: Anti-LGI1, anti-NMDAR, and anti-GABABR encephalitis patients with new-onset seizures were included. Medical information about disease course, AEDs and immunotherapies used, effects, and side effects were collected. Outcome measures were (1) seizure freedom while using AEDs or immunotherapy, (2) days to seizure freedom from start of AEDs or immunotherapy, and (3) side effects.
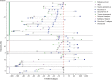
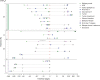
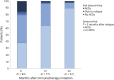
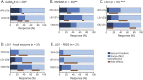
Results: Of 153 patients with autoimmune encephalitis (AIE) (53 LGI1, 75 NMDAR, 25 GABABR), 72% (n = 110) had epileptic seizures, and 89% reached seizure freedom. At least 53% achieved seizure freedom shortly after immunotherapy, and 14% achieved seizure freedom while using only AEDs (p < 0.0001). This effect was similar in all types (p = 0.0001; p = 0.0005; p = 0.013, respectively). Median time to seizure freedom from AEDs start was 59 days (interquartile range [IQR] 27-160), and 28 days from start of immunotherapy (IQR 9-71, p < 0.0001). Side effects were psychotic behavior and suicidal thoughts by the use of levetiracetam, and rash by the use of carbamazepine. Carbamazepine was more effective than levetiracetam in reducing seizures in anti-LGI1 encephalitis (p = 0.031). Only 1 patient, of 86 surviving patients, developed epilepsy after resolved encephalitis.
Conclusion: Epilepsy after resolved encephalitis was rare in our cohort of patients with AIE treated with immunotherapy. In addition, seizure freedom is achieved faster and more frequently after immunotherapy. Therefore, AEDs should be considered as add-on treatment, and similar to treatment of other encephalitis symptoms, immunotherapy is crucial.
Copyright © 2019 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures

Comment in
-
Recognizing autoimmune encephalitis as a cause of seizures: Treating cause and not effect.Neurology. 2019 May 7;92(19):877-878. doi: 10.1212/WNL.0000000000007444. Epub 2019 Apr 12. Neurology. 2019. PMID: 30979858 No abstract available.
-
Putting a Band-Aid on a Broken Leg: Antiseizure Medications Are Inferior to Immune Therapies in Autoimmune Epilepsy.Epilepsy Curr. 2019 Sep;19(5):302-304. doi: 10.1177/1535759719868690. Epub 2019 Aug 22. Epilepsy Curr. 2019. PMID: 31436112 Free PMC article.
References
-
- Irani SR, Michell AW, Lang B, et al. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol 2011;69:892–900. - PubMed
-
- Irani SR, Stagg CJ, Schott JM, et al. Faciobrachial dystonic seizures: the influence of immunotherapy on seizure control and prevention of cognitive impairment in a broadening phenotype. Brain 2013;136:3151–3162. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
