A Novel Rat Model of Patellofemoral Osteoarthritis Due to Patella Baja, or Low-Lying Patella
- PMID: 30979862
- PMCID: PMC6476235
- DOI: 10.12659/MSM.915018
A Novel Rat Model of Patellofemoral Osteoarthritis Due to Patella Baja, or Low-Lying Patella
Abstract
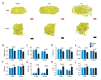
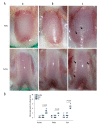
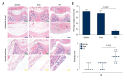
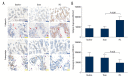
BACKGROUND Patella baja, or patella infera, consists of a low-lying patella that results in a limited range of motion, joint pain, and crepitations. Patellofemoral joint osteoarthritis (PFJOA) is a subtype OA of the knee. This study aimed to develop a reproducible and reliable rat model of PFJOA. MATERIAL AND METHODS Three-month-old female Sprague-Dawley rats (n=24) included a baseline group (n=8) that were euthanized at the beginning of the study. The sham group (n=8), and the patella ligament shortening (PLS) group (n=8) were euthanized and evaluated at ten weeks. The PLS model group (n=8) underwent insertion of a Kirschner wire under the patella tendon to induce patella baja. At ten weeks, the sham group and the PLS group were compared using X-ray imaging, macroscopic appearance, histology, immunohistochemistry, TUNEL staining for apoptosis, and micro-computed tomography (micro-CT). The patella height was determined using the modified Insall-Salvati (MIS) ratio. RESULTS The establishment of the rat model of patella baja in the PLS group at ten weeks was confirmed by X-ray. In the PLS group, patella volume, sagittal length, and cross-sectional area were significantly increased compared with the sham group. The PFJ showed typical lesions of OA, confirmed macroscopically and histologically. Compared with the sham group, in the rat model of PFJOA, there was increased cell apoptosis, and immunohistochemistry showed increased expression of biomarkers of osteoarthritis, compared with the sham group. CONCLUSIONS A rat model of PFJOA was developed that was confirmed by changes in cartilage and subchondral bone.
Conflict of interest statement
None.
Figures





References
-
- Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–223. - PubMed
-
- Davies AP, Vince AS, Shepstone L, et al. The radiologic prevalence of patellofemoral osteoarthritis. Clin Orthop Relat Res. 2002;(402):206–12. - PubMed
-
- Collins NJ, Hinman RS, Menz HB, Crossley KM. Immediate effects of foot orthoses on pain during functional tasks in people with patellofemoral osteoarthritis: A cross-over, proof-of-concept study. Knee. 2017;24:76–81. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

