Steroid receptor coactivator-1 modulates the function of Pomc neurons and energy homeostasis
- PMID: 30979869
- PMCID: PMC6461669
- DOI: 10.1038/s41467-019-08737-6
Steroid receptor coactivator-1 modulates the function of Pomc neurons and energy homeostasis
Abstract
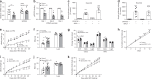
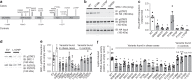
Hypothalamic neurons expressing the anorectic peptide Pro-opiomelanocortin (Pomc) regulate food intake and body weight. Here, we show that Steroid Receptor Coactivator-1 (SRC-1) interacts with a target of leptin receptor activation, phosphorylated STAT3, to potentiate Pomc transcription. Deletion of SRC-1 in Pomc neurons in mice attenuates their depolarization by leptin, decreases Pomc expression and increases food intake leading to high-fat diet-induced obesity. In humans, fifteen rare heterozygous variants in SRC-1 found in severely obese individuals impair leptin-mediated Pomc reporter activity in cells, whilst four variants found in non-obese controls do not. In a knock-in mouse model of a loss of function human variant (SRC-1L1376P), leptin-induced depolarization of Pomc neurons and Pomc expression are significantly reduced, and food intake and body weight are increased. In summary, we demonstrate that SRC-1 modulates the function of hypothalamic Pomc neurons, and suggest that targeting SRC-1 may represent a useful therapeutic strategy for weight loss.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- MC_UU_12012/1/MRC_/Medical Research Council/United Kingdom
- RG/13/13/30194/BHF_/British Heart Foundation/United Kingdom
- P01 DK113954/DK/NIDDK NIH HHS/United States
- R01 DK101379/DK/NIDDK NIH HHS/United States
- P01 DK059820/DK/NIDDK NIH HHS/United States
- R01 HD007857/HD/NICHD NIH HHS/United States
- R01 DK114279/DK/NIDDK NIH HHS/United States
- P30 CA125123/CA/NCI NIH HHS/United States
- K99 DK107008/DK/NIDDK NIH HHS/United States
- R01 DK117281/DK/NIDDK NIH HHS/United States
- R01 DK093587/DK/NIDDK NIH HHS/United States
- R01 CA193455/CA/NCI NIH HHS/United States
- RG/18/13/33946/BHF_/British Heart Foundation/United Kingdom
- R00 DK107008/DK/NIDDK NIH HHS/United States
- MC_UU_00014/1/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- R01 CA112403/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

