Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001
- PMID: 30980071
- PMCID: PMC6637370
- DOI: 10.1093/annonc/mdz131
Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001
Abstract
Background: In the ongoing phase I PROFILE 1001 study, crizotinib showed antitumor activity in patients with ROS1-rearranged advanced non-small-cell lung cancer (NSCLC). Here, we present updated antitumor activity, overall survival (OS) and safety data (additional 46.2 months follow-up) for patients with ROS1-rearranged advanced NSCLC from PROFILE 1001.
Patients and methods: ROS1 status was determined by FISH or reverse transcriptase-polymerase chain reaction. All patients received crizotinib at a starting dose of 250 mg twice daily.
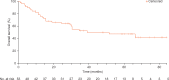
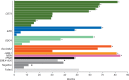
Results: Fifty-three patients received crizotinib, with a median duration of treatment of 22.4 months. At data cut-off, treatment was ongoing in 12 patients (23%). The objective response rate (ORR) was 72% [95% confidence interval (CI), 58% to 83%], including six confirmed complete responses and 32 confirmed partial responses; 10 patients had stable disease. Responses were durable (median duration of response 24.7 months; 95% CI, 15.2-45.3). ORRs were consistent across different patient subgroups. Median progression-free survival was 19.3 months (95% CI, 15.2-39.1). A total of 26 deaths (49%) occurred (median follow-up period of 62.6 months), and of the remaining 27 patients (51%), 14 (26%) were in follow-up at data cut-off. Median OS was 51.4 months (95% CI, 29.3 to not reached) and survival probabilities at 12, 24, 36, and 48 months were 79%, 67%, 53%, and 51%, respectively. No correlation was observed between OS and specific ROS1 fusion partner. Treatment-related adverse events (TRAEs) were mainly grade 1 or 2, per CTCAE v3.0. There were no grade ≥4 TRAEs and no TRAEs associated with permanent discontinuation. No new safety signals were reported with long-term crizotinib treatment.
Conclusions: These findings serve as a new benchmark for OS in ROS1-rearranged advanced NSCLC, and continue to show the clinically meaningful benefit and safety of crizotinib in this molecular subgroup.
Trial registration number: ClinicalTrials.gov identifier NCT00585195.
Keywords: ROS1; crizotinib; non-small-cell lung cancer; overall survival.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Society for Medical Oncology.
Figures
References
-
- Rikova K, Guo A, Zeng Q. et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 2007; 131(6): 1190–1203. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

