Higher Reliance on Glycolysis Limits Glycolytic Responsiveness in Degenerating Glaucomatous Optic Nerve
- PMID: 30980229
- PMCID: PMC6728180
- DOI: 10.1007/s12035-019-1576-4
Higher Reliance on Glycolysis Limits Glycolytic Responsiveness in Degenerating Glaucomatous Optic Nerve
Abstract
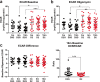
Metabolic dysfunction accompanies neurodegenerative disease and aging. An important step for therapeutic development is a more sophisticated understanding of the source of metabolic dysfunction, as well as to distinguish disease-associated changes from aging effects. We examined mitochondrial function in ex vivo aging and glaucomatous optic nerve using a novel approach, the Seahorse Analyzer. Optic nerves (ON) from the DBA/2J mouse model of glaucoma and the DBA/2-Gpnmb+ control strain were isolated, and oxygen consumption rate (OCR) and extracellular acidification rate (ECAR), the discharge of protons from lactate release or byproducts of substrate oxidation, were measured. The glial-specific aconitase inhibitor fluorocitrate was used to limit the contribution of glial mitochondria to OCR and ECAR. We observed significant decreases in maximal respiration, ATP production, and spare capacity with aging. In the presence of fluorocitrate, OCR was higher, with more ATP produced, in glaucoma compared to aged ON. However, glaucoma ON showed lower maximal respiration. In the presence of fluorocitrate and challenged with ATPase inhibition, glaucoma ON was incapable of further upregulation of glycolysis to compensate for the loss of oxidative phosphorylation. Inclusion of 2-deoxyglucose as a substrate during ATPase inhibition indicated a significantly higher proportion of ECAR was derived from TCA cycle substrate oxidation than glycolysis in glaucoma ON. These data indicate that glaucoma axons have limited ability to respond to increased energy demand given their lower maximal respiration and inability to upregulate glycolysis when challenged. The higher ATP output from axonal mitochondria in glaucoma optic nerve compensates for this lack of resiliency but is ultimately inadequate for continued function.
Keywords: DBA/2J; Fluorocitrate; Glaucoma; Mitochondria; Optic nerve; Seahorse analyzer.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

