Direct oral anticoagulants versus vitamin K antagonists after recent ischemic stroke in patients with atrial fibrillation
- PMID: 30980560
- PMCID: PMC6563449
- DOI: 10.1002/ana.25489
Direct oral anticoagulants versus vitamin K antagonists after recent ischemic stroke in patients with atrial fibrillation
Abstract
Objective: We compared outcomes after treatment with direct oral anticoagulants (DOACs) and vitamin K antagonists (VKAs) in patients with atrial fibrillation (AF) and a recent cerebral ischemia.
Methods: We conducted an individual patient data analysis of seven prospective cohort studies. We included patients with AF and a recent cerebral ischemia (<3 months before starting oral anticoagulation) and a minimum follow-up of 3 months. We analyzed the association between type of anticoagulation (DOAC versus VKA) with the composite primary endpoint (recurrent ischemic stroke [AIS], intracerebral hemorrhage [ICH], or mortality) using mixed-effects Cox proportional hazards regression models; we calculated adjusted hazard ratios (HRs) with 95% confidence intervals (95% CIs).
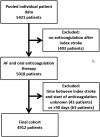
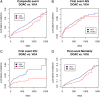
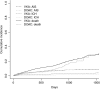
Results: We included 4,912 patients (median age, 78 years [interquartile range {IQR}, 71-84]; 2,331 [47.5%] women; median National Institute of Health Stroke Severity Scale at onset, 5 [IQR, 2-12]); 2,256 (45.9%) patients received VKAs and 2,656 (54.1%) DOACs. Median time from index event to starting oral anticoagulation was 5 days (IQR, 2-14) for VKAs and 5 days (IQR, 2-11) for DOACs (p = 0.53). There were 262 acute ischemic strokes (AISs; 4.4%/year), 71 intracranial hemorrrhages (ICHs; 1.2%/year), and 439 deaths (7.4%/year) during the total follow-up of 5,970 patient-years. Compared to VKAs, DOAC treatment was associated with reduced risks of the composite endpoint (HR, 0.82; 95% CI, 0.67-1.00; p = 0.05) and ICH (HR, 0.42; 95% CI, 0.24-0.71; p < 0.01); we found no differences for the risk of recurrent AIS (HR, 0.91; 95% CI, 0.70-1.19; p = 0.5) and mortality (HR, 0.83; 95% CI, 0.68-1.03; p = 0.09).
Interpretation: DOAC treatment commenced early after recent cerebral ischemia related to AF was associated with reduced risk of poor clinical outcomes compared to VKA, mainly attributed to lower risks of ICH. ANN NEUROL 2019;85:823-834.
© 2019 The Authors. Annals of Neurology published by Wiley Periodicals, Inc. on behalf of American Neurological Association.
Conflict of interest statement
The following companies manufacture drugs involved in this study: Bayer (BY, rivaroxaban), Boehringer Ingelheim (BI, dabigatran), Pfizer/Bristol Meyer Squibb (PB, apixaban), and Daiichi Sankyo (DS, edoxaban). D.J.S.: scientific advisory boards: BY and PB. M.P.: honoraria as a member of the speaker bureau of BI, BY, and PB. M.K.: speaker honoraria from DS, BY, and PB. K.M. speaker honoraria from BY, travel grant from PB. L.H.B.: consultancy or advisory board fees or speaker's honoraria from BY, PB. K.W.M.: participated in advisory boards for DS and BY; member of trial steering committee for BY‐sponsored NAVIGATE‐ESUS trial. G.T.: scientific advisory boards: BY, DS, and BI. S.A.: speaker honoraria from DS, BI, and BMS/PB. H.Y.: speaker honoraria from DS, BY, BI, and BMS/PB. Research funding: PB. S.S.: employment by BY HealthCare Pharmaceuticals’ Japanese subsidiary, BY Yakuhin, from January 2018 (all his contributions to this study were made during his employment at the National Cerebral and Cardiovascular Center). N.P.: advisory boards BI, BY, and DS. K.T.: speaker honoraria from DS, BY, BI, and PB. P.A.L.: scientific advisory boards: BY, DS, and BI. Funding for travel or speaker honoraria: BY and BI. Research funding: BI. D.J.W.: speaking honoraria: BY. S.T.E.: funding for travel or speaker honoraria: BY and BI. Scientific advisory boards: BY, BI, and PB. Educational grant from PB. G.M.D.M.: travel honoraria: BY; speaker honoraria: PB.
Figures

References
-
- Secondary prevention in non‐rheumatic atrial fibrillation after transient ischaemic attack or minor stroke . EAFT (European Atrial Fibrillation Trial) Study Group. Lancet 1993;342:1255–1262. - PubMed
-
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet 2014;383:955–962. - PubMed
-
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–1151. - PubMed

