Relationship between patient characteristics and serum etonogestrel concentrations in contraceptive implant users
- PMID: 30980827
- PMCID: PMC6589369
- DOI: 10.1016/j.contraception.2019.03.045
Relationship between patient characteristics and serum etonogestrel concentrations in contraceptive implant users
Abstract
Objective: To determine whether serum etonogestrel concentrations in contraceptive implant users are associated with certain individual patient characteristics.
Study design: We enrolled reproductive-age women using etonogestrel contraceptive implants between 12-36 months duration and measured a single serum etonogestrel concentration. Participants also completed a questionnaire about demographics.
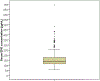
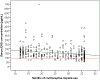
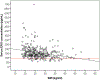
Results: We enrolled 350 participants; median age was 22.5 years (range 18.0-39.1), median months of implant use was 26.0 (range 12.0-36.0), and median body mass index was 25.7 kg/m2 (range 18.5-52.0). Our study population was primarily white/Caucasian (46.6% [163/350]) and Hispanic/Latina ethnicity (51.4% [180/350]). The median serum etonogestrel concentration was 137.4 pg/ml and etonogestrel concentrations varied 12.4 fold in the population (range 55.8-695.1 pg/ml). Using forward stepwise linear regression, months of implant use (β=-1.74, p<.001) and body mass index (β=-3.10, p<.001) were both significantly associated with decreased serum etonogestrel concentration with Black/African American race as a positive effect modifier (β=18.24, p=.099); R-squared for the model=0.13.
Conclusions: Individuals demonstrated a wide variability in serum etonogestrel concentrations, which can potentially affect side-effect profiles and efficacy. Increasing body mass index and longer duration of implant use were associated with small decreases in serum etonogestrel concentrations, while self-reported Black/African American race was associated with a non-significant increase. Despite these findings, most of etonogestrel variability was unaccounted for, suggesting that other clinical, pharmacologic, and genetic factors contributing to variability in etonogestrel concentrations remain to be determined.
Implications: Although increases in body mass index are associated with lower etonogestrel levels in contraceptive implant users, the majority of women will maintain serum concentrations that consistently suppress ovulation. Furthermore, certain patient characteristics can only explain a small portion (13%) of the variability in serum etonogestrel levels among contraceptive implant users.
Trial registration: ClinicalTrials.gov NCT03092037.
Keywords: Body mass index; Contraceptive implant; Etonogestrel; Pharmacokinetics.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests
☐ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
☒The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Figures
Similar articles
-
The effect of carbamazepine on etonogestrel concentrations in contraceptive implant users.Contraception. 2017 Jun;95(6):571-577. doi: 10.1016/j.contraception.2017.03.004. Epub 2017 Mar 10. Contraception. 2017. PMID: 28288788
-
Pharmacokinetics of the etonogestrel contraceptive implant in obese women.Am J Obstet Gynecol. 2012 Aug;207(2):110.e1-6. doi: 10.1016/j.ajog.2012.05.002. Epub 2012 May 8. Am J Obstet Gynecol. 2012. PMID: 22717269
-
Comparison of plasma etonogestrel concentrations sampled from the contralateral-to-implant and ipsilateral-to-implant arms of contraceptive implant users.Contraception. 2020 Dec;102(6):403-405. doi: 10.1016/j.contraception.2020.08.011. Epub 2020 Aug 26. Contraception. 2020. PMID: 32858051 Free PMC article.
-
The pharmacokinetics and pharmacodynamics of Implanon, a single-rod etonogestrel contraceptive implant.Eur J Contracept Reprod Health Care. 2000 Sep;5 Suppl 2:12-20. Eur J Contracept Reprod Health Care. 2000. PMID: 11246602 Review.
-
Medical eligibility criteria for new contraceptive methods: combined hormonal patch, combined hormonal vaginal ring and the etonogestrel implant.Contraception. 2006 Feb;73(2):134-44. doi: 10.1016/j.contraception.2005.08.002. Epub 2005 Oct 20. Contraception. 2006. PMID: 16413844 Review.
Cited by
-
An exploratory analysis on the influence of genetic variants on weight gain among etonogestrel contraceptive implant users.Contraception. 2020 Sep;102(3):180-185. doi: 10.1016/j.contraception.2020.05.002. Epub 2020 May 12. Contraception. 2020. PMID: 32407811 Free PMC article.
-
An exploratory study on the possible association of serum etonogestrel concentrations with mood concerns and symptoms among contraceptive implant users.Contraception. 2024 Jan;129:110298. doi: 10.1016/j.contraception.2023.110298. Epub 2023 Oct 4. Contraception. 2024. PMID: 37802462 Free PMC article.
-
Applicability of ancestral genotyping in pharmacogenomic research with hormonal contraception.Clin Transl Sci. 2021 Sep;14(5):1713-1718. doi: 10.1111/cts.13014. Epub 2021 May 2. Clin Transl Sci. 2021. PMID: 33650294 Free PMC article.
-
Ectopic pregnancy with etonogestrel implant.BMJ Case Rep. 2021 Nov 16;14(11):e245175. doi: 10.1136/bcr-2021-245175. BMJ Case Rep. 2021. PMID: 34785516 Free PMC article.
-
Variability in repeat serum etonogestrel concentrations among contraceptive implant users during the steady-release pharmacokinetic period.Contraception. 2022 Apr;108:65-68. doi: 10.1016/j.contraception.2021.12.008. Epub 2021 Dec 29. Contraception. 2022. PMID: 34973207 Free PMC article.
References
-
- Hatcher RA, Trussell J, Stewart F, et al. Contraceptive technology. New York: Ardent Media: Inc; 2011.
-
- Abma JC, Martinez GM. Sexual Activity and Contraceptive Use Among Teenagers in the United States, 2011–2015. National health statistics reports. 2017:1–23. - PubMed
-
- Daniels K, Daugherty J, Jones J. Current contraceptive status among women aged 15–44: United States, 2011–2013. NCHS data brief. 2014:1–8. - PubMed
-
- Le J, Tsourounis C. Implanon: a critical review. The Annals of pharmacotherapy. 2001;35:329–36. - PubMed
-
- Wenzl R, van Beek A, Schnabel P, Huber J. Pharmacokinetics of Etonogestrel Released From the Contraceptive Implant Implanon. Contraception. 1998;58:283–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

