Vitamin K2-Dependent GGCX and MGP Are Required for Homeostatic Calcium Regulation of Sperm Maturation
- PMID: 30981116
- PMCID: PMC6461585
- DOI: 10.1016/j.isci.2019.03.030
Vitamin K2-Dependent GGCX and MGP Are Required for Homeostatic Calcium Regulation of Sperm Maturation
Abstract
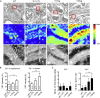
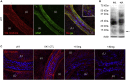
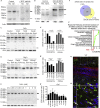
A low-calcium microenvironment is essential for spermatozoa to mature in the epididymis; however, it remains unclear how dysregulation of epididymal luminal calcium is associated with male infertility. Using a warfarin-induced vitamin K2 deficiency rat model, we found that vitamin-K-dependent γ-glutamyl carboxylase (GGCX) and matrix Gla protein (MGP) were essential in extracellular calcium signaling of the intercellular communication required for epididymal sperm maturation. We found that GGCX and MGP co-localized in the vesicular structures of epididymal cells and spermatozoa. Calcium-regulated MGP binds to proteins in a biphasic manner; sub-millimolar calcium enhances, whereas excessive calcium inhibits, the binding. Bioinformatic analysis of the calcium-dependent MGP-bound proteome revealed that vesicle-mediated transport and membrane trafficking underlie the intercellular communication networks. We also identified an SNP mutation, rs699664, in the GGCX gene of infertile men with asthenozoospermia. Overall, we revealed that the GGCX-MGP system is integrated with the intercellular calcium signaling to promote sperm maturation.
Keywords: Cell Biology; Developmental Biology; Specialized Functions of Cells.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Balercia G., Moretti S., Vignini A., Magagnini M., Mantero F., Boscaro M., Ricciardo-Lamonica G., Mazzanti L. Role of nitric oxide concentrations on human sperm motility. J. Androl. 2004;25:245–249. - PubMed
-
- Braun R.E. Post-transcriptional control of gene expression during spermatogenesis. Semin. Cell Dev. Biol. 1998;9:483–489. - PubMed
-
- Breton S., Smith P.J., Lui B., Brown D. Acidification of the male reproductive tract by a proton pumping (H+)-ATPase. Nat. Med. 1996;2:470–472. - PubMed
-
- Chen Q., Yan M.H., Cao Z.H., Li X., Zhang Y.F., Shi J.C., Feng G.H., Peng H.Y., Zhang X.D., Zhang Y. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science. 2016;351:397–400. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

