Lentiviral haemopoietic stem/progenitor cell gene therapy for treatment of Wiskott-Aldrich syndrome: interim results of a non-randomised, open-label, phase 1/2 clinical study
- PMID: 30981783
- PMCID: PMC6494976
- DOI: 10.1016/S2352-3026(19)30021-3
Lentiviral haemopoietic stem/progenitor cell gene therapy for treatment of Wiskott-Aldrich syndrome: interim results of a non-randomised, open-label, phase 1/2 clinical study
Abstract
Background: Wiskott-Aldrich syndrome is a rare, life-threatening, X-linked primary immunodeficiency characterised by microthrombocytopenia, infections, eczema, autoimmunity, and malignant disease. Lentiviral vector-mediated haemopoietic stem/progenitor cell (HSPC) gene therapy is a potentially curative treatment that represents an alternative to allogeneic HSPC transplantation. Here, we report safety and efficacy data from an interim analysis of patients with severe Wiskott-Aldrich syndrome who received lentiviral vector-derived gene therapy.
Methods: We did a non-randomised, open-label, phase 1/2 clinical study in paediatric patients with severe Wiskott-Aldrich syndrome, defined by either WAS gene mutation or absent Wiskott-Aldrich syndrome protein (WASP) expression or a Zhu clinical score of 3 or higher. We included patients who had no HLA-identical sibling donor available or, for children younger than 5 years of age, no suitable 10/10 matched unrelated donor or 6/6 unrelated cord blood donor. After treatment with rituximab and a reduced-intensity conditioning regimen of busulfan and fludarabine, patients received one intravenous infusion of autologous CD34+ cells genetically modified with a lentiviral vector encoding for human WAS cDNA. The primary safety endpoints were safety of the conditioning regimen and safety of lentiviral gene transfer into HSPCs. The primary efficacy endpoints were overall survival, sustained engraftment of genetically corrected HSPCs, expression of vector-derived WASP, improved T-cell function, antigen-specific responses to vaccinations, and improved platelet count and mean platelet volume normalisation. This interim analysis was done when the first six patients treated had completed at least 3 years of follow-up. The planned analyses are presented for the intention-to-treat population. This trial is registered with ClinicalTrials.gov (number NCT01515462) and EudraCT (number 2009-017346-32).
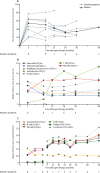
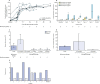
Findings: Between April 20, 2010, and Feb 26, 2015, nine patients (all male) were enrolled of whom one was excluded after screening; the age range of the eight treated children was 1·1-12·4 years. At the time of the interim analysis (data cutoff April 29, 2016), median follow-up was 3·6 years (range 0·5-5·6). Overall survival was 100%. Engraftment of genetically corrected HSPCs was successful and sustained in all patients. The fraction of WASP-positive lymphocytes increased from a median of 3·9% (range 1·8-35·6) before gene therapy to 66·7% (55·7-98·6) at 12 months after gene therapy, whereas WASP-positive platelets increased from 19·1% (range 4·1-31·0) to 76·6% (53·1-98·4). Improvement of immune function was shown by normalisation of in-vitro T-cell function and successful discontinuation of immunoglobulin supplementation in seven patients with follow-up longer than 1 year, followed by positive antigen-specific response to vaccination. Severe infections fell from 2·38 (95% CI 1·44-3·72) per patient-year of observation (PYO) in the year before gene therapy to 0·31 (0·04-1·11) per PYO in the second year after gene therapy and 0·17 (0·00-0·93) per PYO in the third year after gene therapy. Before gene therapy, platelet counts were lower than 20 × 109 per L in seven of eight patients. At the last follow-up visit, the platelet count had increased to 20-50 × 109 per L in one patient, 50-100 × 109 per L in five patients, and more than 100 × 109 per L in two patients, which resulted in independence from platelet transfusions and absence of severe bleeding events. 27 serious adverse events in six patients occurred after gene therapy, 23 (85%) of which were infectious (pyrexia [five events in three patients], device-related infections, including one case of sepsis [four events in three patients], and gastroenteritis, including one case due to rotavirus [three events in two patients]); these occurred mainly in the first 6 months of follow-up. No adverse reactions to the investigational drug product and no abnormal clonal proliferation or leukaemia were reported after gene therapy.
Interpretation: Data from this study show that gene therapy provides a valuable treatment option for patients with severe Wiskott-Aldrich syndrome, particularly for those who do not have a suitable HSPC donor available.
Funding: Italian Telethon Foundation, GlaxoSmithKline, and Orchard Therapeutics.
Copyright © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- Thrasher AJ, Burns SO. WASP: a key immunological multitasker. Nat Rev Immunol. 2010;10:182–192. - PubMed
-
- Ochs HD, Thrasher AJ. The Wiskott-Aldrich syndrome. J Allergy Clin Immunol. 2006;117:725–738. - PubMed
-
- Bosticardo M, Marangoni F, Aiuti A, Villa A, Roncarolo MG. Recent advances in understanding the pathophysiology of Wiskott-Aldrich syndrome. Blood. 2009;113:6288–6295. - PubMed
-
- Sullivan KE, Mullen CA, Blaese RM, Winkelstein JA. A multi-institutional survey of the Wiskott-Aldrich syndrome. J Pediatr. 1994;125:876–885. - PubMed
-
- Dupuis-Girod S, Medioni J, Haddad E. Autoimmunity in Wiskott-Aldrich syndrome: risk factors, clinical features, and outcome in a single-center cohort of 55 patients. Pediatrics. 2003;111:e622–e627. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

