Functional resting-state brain connectivity is accompanied by dynamic correlations of application-dependent [18F]FDG PET-tracer fluctuations
- PMID: 30981858
- PMCID: PMC10673660
- DOI: 10.1016/j.neuroimage.2019.04.034
Functional resting-state brain connectivity is accompanied by dynamic correlations of application-dependent [18F]FDG PET-tracer fluctuations
Abstract
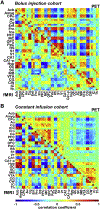
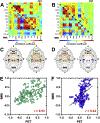
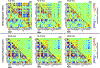
Brain function is characterized by a convolution of various biochemical and physiological processes, raising the interest whether resting-state functional connectivity derived from hemodynamic scales shows underlying metabolic synchronies. Increasing evidence suggests that metabolic connectivity based on glucose consumption associated PET recordings may serve as a marker of cognitive functions and neuropathologies. However, to what extent fMRI-derived resting-state brain connectivity can also be characterized based on dynamic fluctuations of glucose metabolism and how metabolic connectivity is influenced by [18F]FDG pharmacokinetics remains unsolved. Simultaneous PET/MRI measurements were performed in a total of 26 healthy male Lewis rats. Simultaneously to resting-state fMRI scans, one cohort (n = 15) received classical bolus [18F]FDG injections and dynamic PET images were recorded. In a second cohort (n = 11) [18F]FDG was constantly infused over the entire functional PET/MRI scans. Resting-state fMRI and [18F]FDG-PET connectivity was evaluated using a graph-theory based correlation approach and compared on whole-brain level and for a default-mode network-like structure. Further, pharmacokinetic and tracer uptake influences on [18F]FDG-PET connectivity results were investigated based on the different PET protocols. By integrating simultaneous resting-state fMRI and dynamic [18F]FDG-PET measurements in the rat brain, we identified homotopic correlations between both modalities, suggesting an underlying synchrony between hemodynamic processes and glucose consumption. Furthermore, the presence of the prominent resting-state default-mode network-like structure was not only depicted on a functional scale but also from dynamic fluctuations of [18F]FDG. In addition, the present findings demonstrated strong pharmacokinetic and tracer uptake dependencies of [18F]FDG-PET connectivity outcomes. This study highlights the application of dynamic [18F]FDG-PET to study cognitive brain functions and to decode underlying brain networks in the resting-state. Thereby, PET-derived connectivity outcomes indicated strong dependencies on tracer application regimens and subsequent time-varying tracer pharmacokinetics.
Keywords: Connectomics; Resting-state brain networks; Simultaneous PET/fMRI; fPET.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest
The authors declare no conflict of interest.
Figures




References
-
- Aiello M, Salvatore E, Cachia A, Pappata S, Cavaliere C, Prinster A, Nicolai E, Salvatore M, Baron JC, Quarantelli M, 2015. Relationship between simultaneously acquired resting-state regional cerebral glucose metabolism and functional MRI: a PET/MR hybrid scanner study. Neuroimage 113, 111–121. - PubMed
-
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS, 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 34 (4), 537–541. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

