The new genetic landscape of Alzheimer's disease: from amyloid cascade to genetically driven synaptic failure hypothesis?
- PMID: 30982098
- PMCID: PMC6660578
- DOI: 10.1007/s00401-019-02004-0
The new genetic landscape of Alzheimer's disease: from amyloid cascade to genetically driven synaptic failure hypothesis?
Abstract
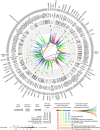
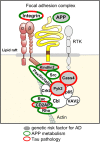
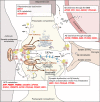
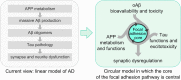
A strong genetic predisposition (60-80% of attributable risk) is present in Alzheimer's disease (AD). In view of this major genetic component, identification of the genetic risk factors has been a major objective in the AD field with the ultimate aim to better understand the pathological processes. In this review, we present how the genetic risk factors are involved in APP metabolism, β-amyloid peptide production, degradation, aggregation and toxicity, innate immunity, and Tau toxicity. In addition, on the basis of the new genetic landscape, resulting from the recent high-throughput genomic approaches and emerging neurobiological information, we propose an over-arching model in which the focal adhesion pathway and the related cell signalling are key elements in AD pathogenesis. The core of the focal adhesion pathway links the physiological functions of amyloid precursor protein and Tau with the pathophysiological processes they are involved in. This model includes several entry points, fitting with the different origins for the disease, and supports the notion that dysregulation of synaptic plasticity is a central node in AD. Notably, our interpretation of the latest data from genome wide association studies complements other hypotheses already developed in the AD field, i.e., amyloid cascade, cellular phase or propagation hypotheses. Genetically driven synaptic failure hypothesis will need to be further tested experimentally within the general AD framework.
Figures

References
-
- Andrew RJ, De Rossi P, Nguyen P, Kowalski HR, Recupero AJ, Guerbette T, et al. Reduction of the expression of the late-onset Alzheimer’s disease (AD) risk-factor BIN1 does not affect amyloid pathology in an AD mouse model. J Biol Chem. 2019;294:4477–4487. doi: 10.1074/jbc.ra118.006379. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

